Dyspnoea is a common symptom of respiratory disease. However, data on its prevalence in general populations and its association with lung function are limited and are mainly from high-income countries. The aims of this study were to estimate the prevalence of dyspnoea across several world regions, and to investigate the association of dyspnoea with lung function.
MethodsDyspnoea was assessed, and lung function measured in 25,806 adult participants of the multinational Burden of Obstructive Lung Disease study. Dyspnoea was defined as ≥2 on the modified Medical Research Council (mMRC) dyspnoea scale. The prevalence of dyspnoea was estimated for each of the study sites and compared across countries and world regions. Multivariable logistic regression was used to assess the association of dyspnoea with lung function in each site. Results were then pooled using random-effects meta-analysis.
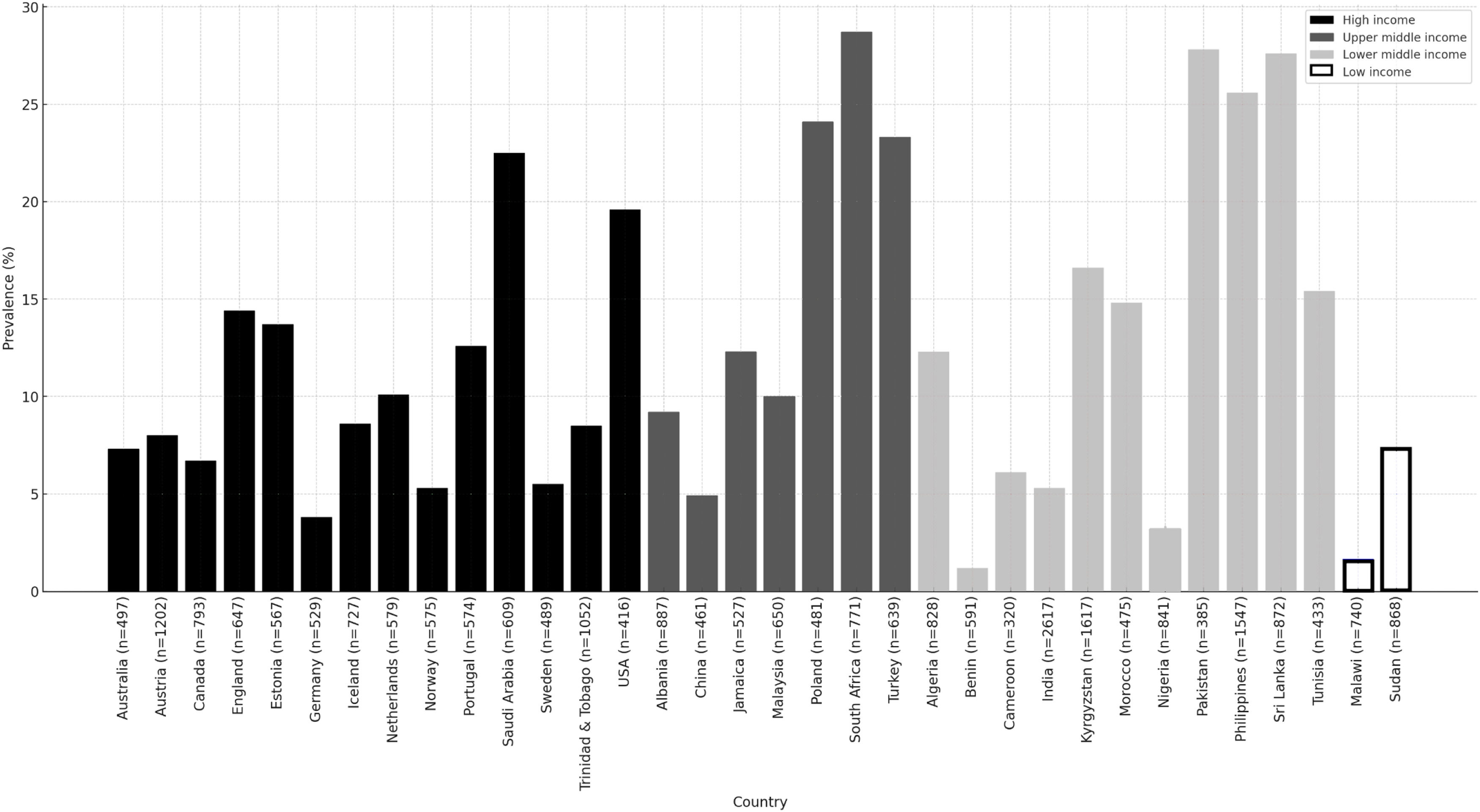
ResultsThe prevalence of dyspnoea varied widely across sites without a clear geographical pattern. The mean prevalence of dyspnoea was 13.7 % (SD=8.2 %), ranging from 0 % in Mysore (India) to 28.8 % in Nampicuan-Talugtug (Philippines). Dyspnoea was strongly associated with both spirometry restriction (FVC1/FVC
ConclusionThe prevalence of dyspnoea varies substantially across the world and is strongly associated with lung function impairment. Using the mMRC scale in epidemiological research should be discussed.
Dyspnoea is a subjective and discomfortable experience of breathlessness.1 It is a symptom associated with various cardiorespiratory diseases2, and is more common among older people, women, smokers, and both over- and underweight people.3–5 Dyspnoea may occur acutely in cases of potentially life-threatening conditions, but it often develops over time as part of a chronic disease.2 It has been shown that dyspnoea is associated with disease severity, reduced health-related quality of life and increased mortality.6,7 Despite its clinical importance, there is still a gap in the literature regarding the burden of dyspnoea in general populations. Prevalence estimates range from 2 to 32 percent with increasing prevalence in older age populations.8–12,5,13 However, these estimates are mainly from Western high-income countries, and little is known about the worldwide variation of dyspnoea prevalence and its determinants.
Grønseth et al. investigated the prevalence of dyspnoea and its association with lung function using data from the multinational Burden of Obstructive Lung Disease (BOLD) study in 15 countries.14 They found a strong geographic variation in dyspnoea prevalence across these countries, most of which are in Europe and North America. In addition, the authors found a strong association of dyspnoea with low forced vital capacity (FVC). This study includes the same 15 study sites as Grønseth et al. and 26 additional sites, from countries across Africa, Asia, Europe and the Caribbean. The aims were to estimate the prevalence of dyspnoea across several world regions, and to improve the understanding of the association between lung function and dyspnoea.
MethodsStudy designThe design of the population-based BOLD study has been published elsewhere.15 Briefly, adults, aged 40 years or older, were recruited in 41 sites across 34 countries. Study sites used either stratified or simple random sampling or cluster sampling for recruitment and sample weights were calculated for each site to improve representation of the general population. Data collection included age, sex, measurements of height and weight as well as pre- and post-bronchodilator spirometry conducted by trained and certified staff. Questionnaires on dyspnoea, comorbidities and potential risk factors were used. All questionnaires were translated in the local languages and administered by adequately trained field staff. All study sites obtained approval from their local ethics committee, and all participants provided informed consent.
DyspnoeaDyspnoea was assessed in all participants using the 5-item modified Medical Research Council (mMRC) dyspnoea scale16: Grade 0 – breathless only with strenuous exercise; Grade 1 – breathless when hurrying on level ground or up a slight hill; Grade 2 – breathless when walking at own pace on the level; Grade 3 – breathless when walking 100 yards or for a few minutes; Grade 4 – too breathless to leave the house or breathless when dressing or undressing. Clinically relevant dyspnoea was defined as grade 2 or above.
Lung functionLung function was measured using spirometry (ndd EasyOne Diagnostic 2001, Zurich, Switzerland). Measurements post bronchodilation were performed after the inhalation of 200 µg of albuterol/salbutamol. The quality of spirometry measurements was assessed based on the American Thoracic Society (ATS) acceptability and reproducibility criteria.17 Spirometric restriction was defined as a post-bronchodilator forced vital capacity (FVC) below the lower limit of normal (LLN), and spirometry airflow obstruction as a post-bronchodilator forced expiratory volume in one second (FEV1)/FVC ratio below LLN. The reference equations for Caucasians from the US National Health and Nutrition Examination Survey (NHANES) III were used to calculate the LLN.18
Statistical analysesAll analyses were performed using SPSS Statistics version 28 (IBM, Armonk, NY, USA), and significance level was set at p < 0.05. First, the prevalence estimates for dyspnoea (mMRC ≥ 2) as well as the prevalence estimates for potential risk factors for each of the 41 study sites were calculated. Prevalence of dyspnoea was estimated per site, pooled for countries with more than one site, and also presented by gross national income (GNI) per capita based on data from the World Bank Group.19
To assess the association of dyspnoea with FVC and FEV1/FVC, logistic regression models adjusted for sex, age, height, and body mass index (BMI) (underweight, ≤18 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2; obese, 30–34.9 kg/m2; severely obese, ≥35 kg/m2) were built. These models also included self-reported smoking status (ever smokers, never smokers), whereby an ever smoker was defined as someone who had smoked >20 packs of cigarettes in a lifetime or >1 cigarette per day for a year. It also included self-reported comorbidities: diabetes, cardiovascular disease (CVD, defined as history of either heart attack or stroke), hypertension, and history of tuberculosis. Each regression model was run within each study site, and then site estimates were pooled using random effects meta-analysis. Heterogeneity across sites was summarised using the I2 statistic. To explore variation in the association between lung function and dyspnoea, stratification of the meta-analysis was conducted by: 1) age group (40 to 59 years, ≥60 years); 2) sex (males, females); 3) smoking status (never smokers, ever smokers); and 4) BMI (normal weight plus overweight; obese plus severely obese).
ResultsCharacteristics of participantsA total of 28,604 participants completed the core questionnaire and provided lung function measurements. Of these, 2798 were excluded as they did not complete the questions on dyspnoea. Therefore, the study population consisted of 25,806 participants. An overview of the participants’ characteristics for each of the 41 study sites can be found in table S1 of the supplementary material. In general, there were slightly more females than males. Mean age ranged from 46.7 years in Mysore (India) to 63.3 years in Lisbon (Portugal). Prevalence of smoking varied substantially from 2.0 % in Seme-Kpodji (Benin) to 67.8 % in Uitsig and Ravensmead (South Africa). Underweight was most prevalent in Nampicuan-Talugtug (Philippines) with 20.4 %, while severe obesity was highest in Riyadh (Saudi Arabia) with 22.0 %. The prevalence rates for comorbidities also varied between study sites. Riyadh in Saudi Arabia had the highest prevalence of diabetes (29.9 %), while arterial hypertension was highest in Lexington (KY, USA) with 49.2 %. CVDs were most prevalent in Tartu (Estonia) with 37.4 %. A history of tuberculosis was not common, with the highest prevalence found in Uitsig and Ravensmead (South Africa) with 15.1 %. Spirometry restriction varied considerably between 8.5 % in Vancouver (Canada) and 79.5 % in Mysore (India). Spirometry airflow obstruction ranged from 3.1 % in Riyadh (Saudi Arabia) to 19.0 % in Uitsig and Ravensmead (South Africa).
Prevalence of dyspnoeaFig. 1 shows the prevalence of dyspnoea for all 34 countries of the BOLD study, clustered by their GNI per capita. The prevalence of dyspnoea ranged from 0.0 % in Mysore (India) to 28.8 % in Nampicuan-Talugtug (Philippines). The mean prevalence of dyspnoea for all sites combined was 13.7 % (SD=8.2 %). By country, the lowest prevalence of dyspnoea was found in Benin and Malawi, whereas South Africa and Pakistan showed the highest prevalence.
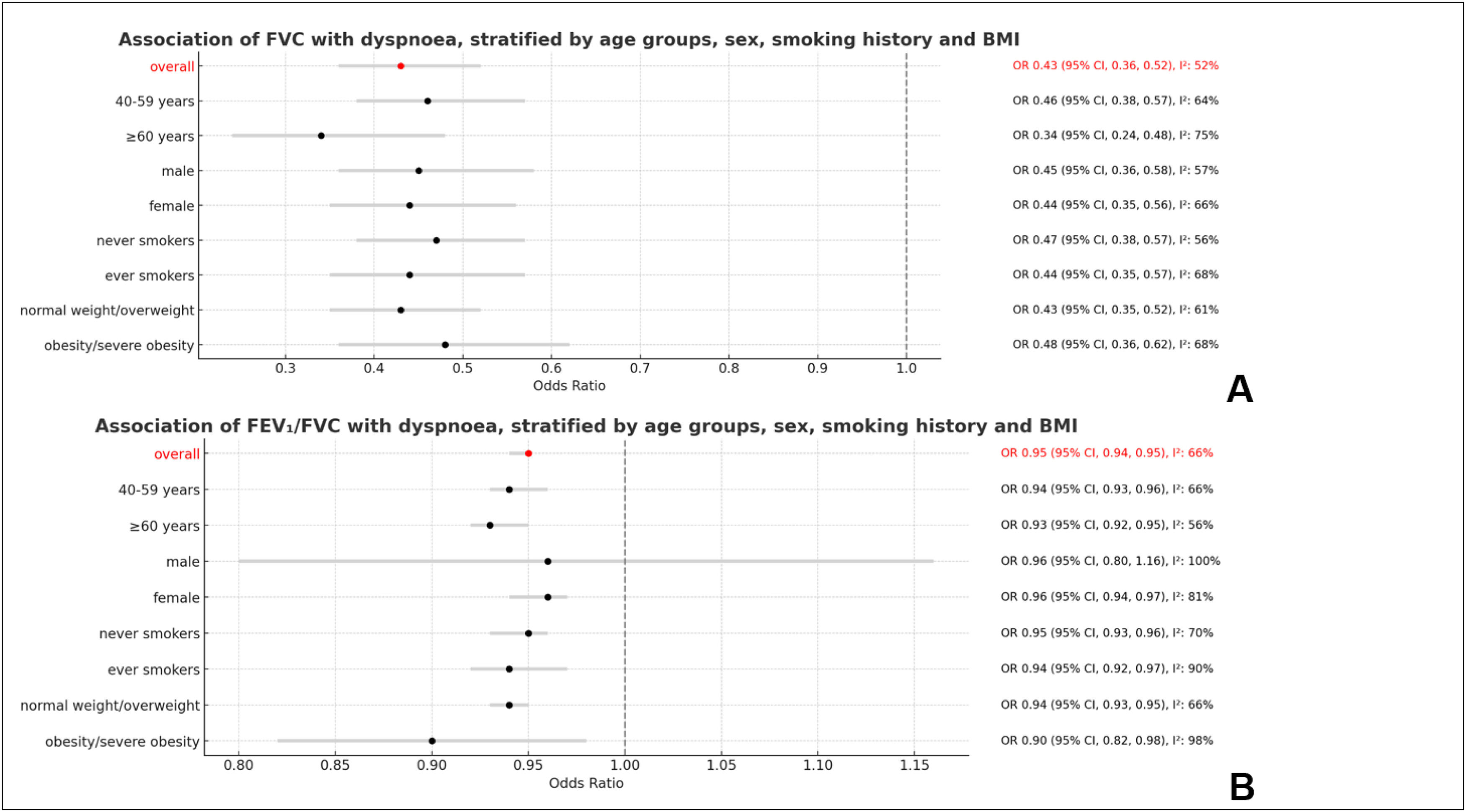
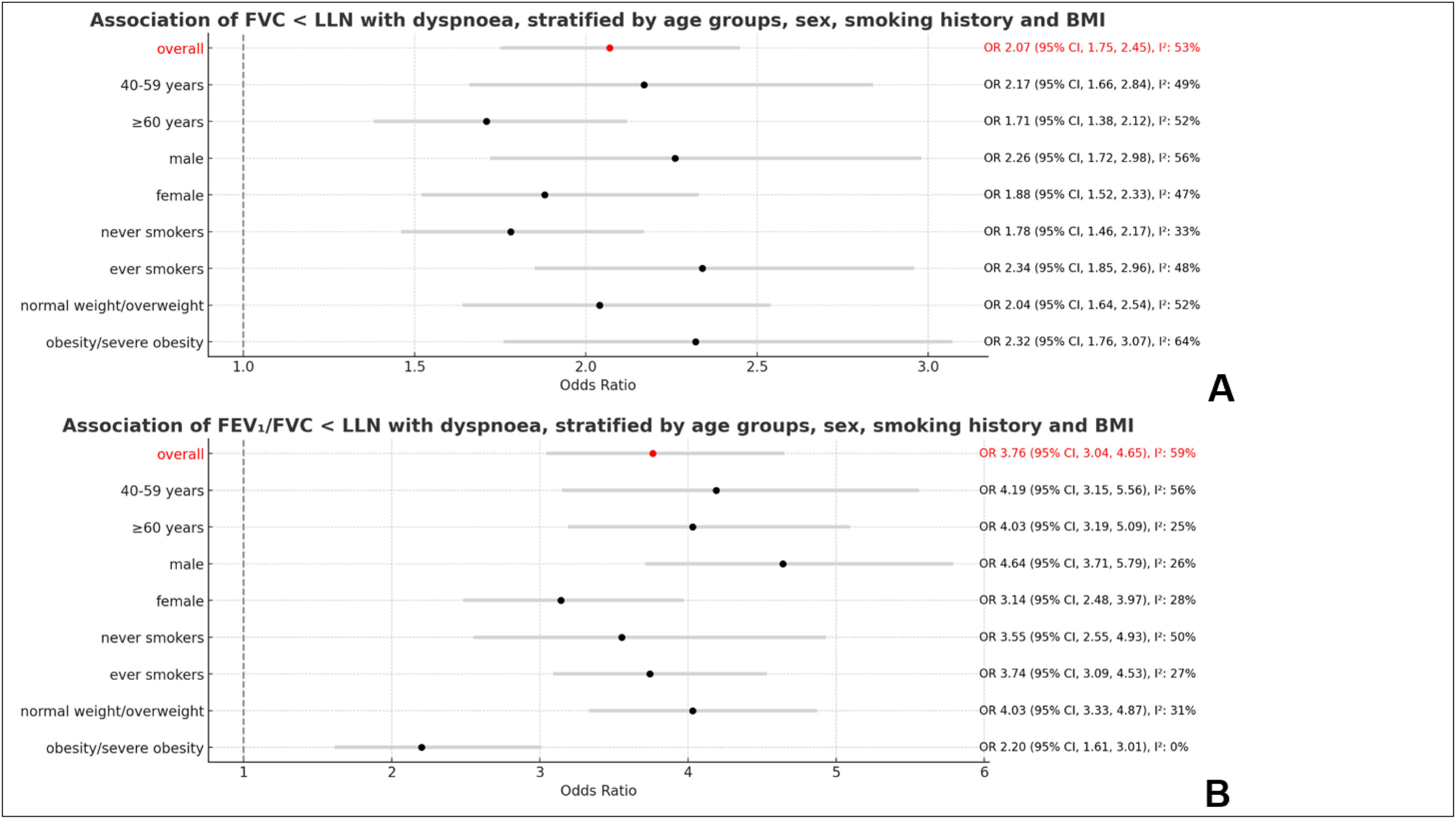
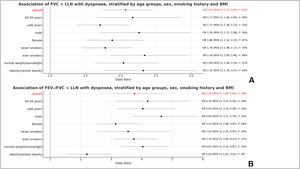
Association of dyspnoea and lung functionDyspnoea was associated with FVC (per 1 litre) (OR 0.43, 95 % CI 0.36, 0.52; I2 = 52 %) and FEV1/FVC (per 1 %) (OR 0.95, 95 % CI 0.94, 0.95; I2 = 66 %) as shown in Fig. 2. Dyspnoea was also strongly associated with both spirometry restriction (OR 2.07, 95 % CI 1.75, 2.45; I2 = 53 %) and spirometric airflow obstruction (OR 3.76, 95 % CI 3.04, 4.65; I2 = 59 %) as presented in Fig. 3.
These associations were independent of sex, age, height, smoking history, and BMI. Stratified meta-analyses did not show any significant sex or age differences in the association of dyspnoea with lung function parameters. This association was also not significantly different between ever smokers and never smokers. The only statistically significant difference in the association of dyspnoea with lung function was found between obese and non-obese participants, whereby the association of dyspnoea with spirometry airflow obstruction was weaker among obese (OR 2.20, 95 % CI 1.61, 3.01) than among non-obese (OR 4.03, 95 % CI 3.33, 4.87) (Fig. 3).
DiscussionIn this population-based study, the prevalence of dyspnoea varied widely across several world regions. However, there was no clear pattern that could explain this variation. The association of dyspnoea with spirometry restriction was confirmed and did not vary due to sex, age, smoking or BMI. The association of dyspnoea with spirometry airflow obstruction was similarly unaffected by sex, age, or smoking status. However, dyspnoea was more strongly associated with spirometry airflow obstruction among normal weight/overweight participants.
The most striking finding of our analyses was the wide variation of dyspnoea prevalence. The mean prevalence was slightly higher than in previous population-based studies20 which possibly can be explained by the study population that only included participants of 40 years or older and therefore not fully reflects the adult general population. However, the wide range of dyspnoea prevalence (0.0 % to 28.8 %) is surprising. This might be explained by the way dyspnoea was assessed. To date, no consensus on how to examine dyspnoea in a standardised way exists.1 The mMRC scale used in this study is the most widely used tool in population-based studies20 but may not validly measure the complex symptom dyspnoea, as the mMRC scale only measures breathlessness in relation to physical activity. It has also been mentioned in previous publications that the reporting of subjective symptoms is influenced by cultural or linguistic differences that cannot completely be diminished even by using a standardised assessment tool.21 However, it has also been suggested that dichotomising the mMRC scale to define clinically relevant dyspnoea, might help in reducing some of this heterogeneity.22 We followed this approach in our study, which might explain why we reported a prevalence estimate of 0.0 % in Mysore (India). However, without dichotomising, the prevalence of dyspnoea including mMRC grade 1 in this site would have been 0.1 % which still seems very low and highlights the need for a different way of examining dyspnoea. Multidimensional dyspnoea assessment tools like the Multidimensional Dyspnoea Profile or the Dyspnoea-12 questionnaire have shown to have better validity in measuring clinically relevant dyspnoea.23 These tools are currently mainly used in clinical research but their relevance for epidemiological research on dyspnoea should be discussed.
Besides the tool for assessing dyspnoea, other factors might explain the variation in dyspnoea prevalence. When analysing the sites with high dyspnoea prevalence (Nampicuan-Talugtug, Philippines; Karachi, Pakistan), no clear pattern regarding potential risk factors could be identified. The sites with the lowest prevalence estimates for dyspnoea (Mysore, India; Seme-Kpodji, Benin; Chikwawa, Malawi) shared a very low prevalence in smoking and cardiovascular disease. It is therefore reasonable to conclude that smoking and smoking-related diseases (CVDs, spirometry airflow obstruction) might be associated with dyspnoea as previously reported.8 However, in this study, it was not possible to identify a single independent parameter that can be used to predict dyspnoea prevalence across all study sites, but the combination of certain risk factors might explain some of the dyspnoea prevalence for each site.
As this study includes a wide range of low-, middle- and high-income countries, it was reasonable to examine whether geographical variation of dyspnoea prevalence was related to economic differences. A country's GNI per capita might lead to variations in lifestyle-related risk factors for dyspnoea such as smoking or obesity. We therefore used data from the World Bank to cluster BOLD study sites based on GNI per capita. However, no clear association between gross national income and dyspnoea prevalence could be established, with some sites in high-income countries (e.g. Hannover, Germany; Bergen, Norway) showing a very low prevalence of dyspnoea and others (e.g. Lexington, KY, USA; Riyadh, Saudi Arabia) showing considerably higher prevalence. We therefore did not conduct any further analyses. The findings suggest that the global variation in dyspnoea prevalence is not sufficiently explained by economic factors.
Another aim of this study was to assess the association of dyspnoea with abnormal lung function defined as either FVC1/FVC2,24,25 the findings of this study suggest that these factors do not have an impact on the association between dyspnoea and lung function. Our results further imply that obesity modifies the association between dyspnoea and spirometry airflow obstruction, with this association being weaker amongst obese people. It has been hypothesised that obesity, to a certain extent, might improve respiratory mechanics in patients with obstructive lung disease, especially during exercise, due to a better length-tension-relationship of the diaphragm.26,27 However, obesity is also considered an independent risk factor for dyspnoea, especially in individuals with low FVC.28 This means that although obesity might positively modify the association of dyspnoea and airflow obstruction, the beneficial effects on breathlessness on exertion might be levelled out by the negative impact of obesity itself.
We used the NHANES III reference equations for Caucasians to calculate the LLN for all participants in this study, regardless of their location or ethnicity. This might have led to very high prevalence estimates for spirometry restriction in some study sites. However, as association analyses were conducted within each site and only then meta-analysed, it is unlikely that our findings would have been different if local equations had been used. Using ethnic-specific reference equations for lung function parameters has been challenged recently.29
Besides some already mentioned limitations such as the use of the mMRC scale, the reference equations and the participants’ age compared to other population-based studies, this study has also strengths. It is a population-based study including 41 sites from several world regions. This adds valuable information to the understanding of epidemiological characteristics of dyspnoea. The standardisation of the BOLD study protocol, including the administration of the same questionnaires, use of same model of spirometers and spirometry quality control, is another strength as it allows for good comparability across sites.
ConclusionThis study shows that dyspnoea prevalence varies substantially across sites, countries, and world regions. No single predictor explaining this variation in dyspnoea prevalence could be identified. This study further shows that dyspnoea is strongly associated with impaired lung function. As the global variation of dyspnoea could not be fully explained by this study, further research is needed to investigate predictors for dyspnoea prevalence. This study further highlights the urgent need for valid tools for dyspnoea assessment in epidemiological research.
Author contributionsAM, EFMW, DJAJ and AFSA conceived the study. Under the supervision of EFMW, DJAJ, and AFSA, AM performed data analysis and prepared the initial draft. All authors provided critical revision of the manuscript, as well as read and approved the final manuscript.
FundingWellcome Trust grant (085790/Z/08/Z). The funders of the study did not contribute to the study design, data collection, data analysis or writing of the manuscript.
Use of generative AINone.
We thank all participants and field workers for their time and effort put into this study.
BOLD (Burden of Obstructive Lung Disease) Collaborative Research Group members: Albania: Hasan Hafizi (principal investigator [PI]), Anila Aliko, Donika Bardhi, Holta Tafa, Natasha Thanasi, Arian Mezini, Alma Teferici, Dafina Todri, Jolanda Nikolla, and Rezarta Kazasi (Tirana University Hospital Shefqet Ndroqi, Albania); Algeria: Hamid Hacene Cherkaski (PI), Amira Bengrait, Tabarek Haddad, Ibtissem Zgaoula, Maamar Ghit, Abdelhamid Roubhia, Soumaya Boudra, Feryal Atoui, Randa Yakoubi, Rachid Benali, Abdelghani Bencheikh, and Nadia Ait-Khaled (Faculte de M edecine Annaba, Service de Epidemiologie et M edecine Preventive, El Hadjar, Algeria); Australia: Christine Jenkins (PI), Guy Marks (PI), Tessa Bird, Paola Espinel, Kate Hardaker, and Brett Toelle (Woolcock Institute of Medical Research, Sydney, Australia); Austria: Michael Studnicka (PI), Torkil Dawes, Bernd Lamprecht, and Lea Schirhofer (Department of Pulmonary Medicine, Paracelsus Medical University, Salzburg, Austria); Bangladesh: Akramul Islam (PI), Syed Masud Ahmed (Co-PI), Shayla Islam, Qazi Shafayetul Islam, Mesbah-Ul-Haque, Tridib Roy Chowdhury, Sukantha Kumar Chatterjee, Dulal Mia, Shyamal Chandra Das, Mizanur Rahman, Nazrul Islam, Shahaz Uddin, Nurul Islam, Luiza Khatun, Monira Parvin, Abdul Awal Khan, and Maidul Islam (James P. Grant School of Public Health, BRAC [Building Resources Across Communities] University, Institute of Global Health, Dhaka, Bangladesh); Benin: Herve Lawin (PI), Arsene Kpangon, Karl Kpossou, Gildas Agodokpessi, Paul Ayelo, and Benjamin Fayomi (Unit of Teaching and Research in Occupational and Environmental Health, University of Abomey Calavi, Cotonou, Benin); Cameroon: Bertrand Mbatchou (PI) and Atongno Humphrey Ashu (Douala General Hospital, Douala, Cameroon); Canada: Wan C. Tan (PI) and Wen Wang (iCapture Center for Cardiovascular and Pulmonary Research, University of British Columbia, Vancouver, BC, Canada); China: NanShan Zhong (PI), Shengming Liu, Jiachun Lu, Pixin Ran, Dali Wang, Jin-ping Zheng, and Yumin Zhou (Guangzhou Institute of Respiratory Health, First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China); Estonia: Rain Jogi (PI), Hendrik Laja, Katrin Ulst, Vappu ∼ Zobel, and Toomas-Julius Lill (Lung Clinic, Tartu University Hospital, Tartu, Estonia); Gabon: Ayola Akim Adegnika (PI) (Centre de Recherches Medicale de Lambarene, Lambarene, Gabon); Germany: Tobias Welte (PI), Isabelle Bodemann, Henning Geldmacher, and Alexandra Schweda-Linow (Department of Pneumology, Hannover Medical School and German Center of Lung Research, Hannover, Germany); Iceland: Thorarinn Gislason (PI), Bryndis Benedikdtsdottir, Kristin Jorundsdottir, Lovisa Gudmundsdottir, Sigrun Gudmundsdottir, and Gunnar Gudmundsson (Department of Allergy, Respiratory Medicine, and Sleep, Landspitali University Hospital, Reykjavik, Iceland); India: Mahesh Rao (PI) (JSS Medical College, Mysuru, India); Parvaiz A. Koul (PI), Sajjad Malik, Nissar A. Hakim, and Umar Hafiz Khan (Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K, India); Rohini Chowgule (PI), Vasant Shetye, Jonelle Raphael, Rosel Almeda, Mahesh Tawde, Rafiq Tadvi, Sunil Katkar, Milind Kadam, Rupesh Dhanawade, and Umesh Ghurup (Indian Institute of Environmental Medicine, Mumbai, India); Sanjay Juvekar (PI), Siddhi Hirve, Somnath Sambhudas, Bharat Chaidhary, Meera Tambe, Savita Pingale, Arati Umap, Archana Umap, Nitin Shelar, Sampada Devchakke, Sharda Chaudhary, Suvarna Bondre, Savita Walke, Ashleshsa Gawhane, Anil Sapkal, Rupali Argade, and Vijay Gaikwad (Vadu Health and Demographic Surveillance System, King Edward Memorial Hospital Research Centre Pune, Pune India); Sundeep Salvi (PI), Bill Brashier, Jyoti Londhe, and Sapna Madas (Chest Research Foundation, Pune India); Jamaica: Althea Aquart-Stewart (PI) and Akosua Francia Aikman (University of the West Indies, Kingston, Jamaica); Kyrgyzstan: Talant M. Sooronbaev (PI), Bermet M. Estebesova, Meerim Akmatalieva, Saadat Usenbaeva, Jypara Kydyrova, Eliza Bostonova, Ulan Sheraliev, Nuridin Marajapov, Nurgul Toktogulova, Berik Emilov, Toktogul Azilova, Gulnara Beishekeeva, Nasyikat Dononbaeva, and AijamalTabyshova (Pulmonology and Allergology Department, National Centre of Cardiology and Internal Medicine, Bishkek, Kyrgyzstan); Malawi: Kevin Mortimer (PI), Wezzie Nyapigoti, Ernest Mwangoka, Mayamiko Kambwili, Martha Chipeta, Gloria Banda, Suzgo Mkandawire, and Justice Banda (the Malawi Liverpool Wellcome Trust, Blantyre, Malawi); Malaysia: Li-Cher Loh (PI), Abdul Rashid, and Siti Sholehah (Royal College of Surgeons in Ireland and University College Dublin Malaysia Campus); Morocco: Mohamed C. Benjelloun (PI), Chakib Nejjari, Mohamed Elbiaze, and Karima El Rhazi (Laboratoire d'epid emiologie, Recherche Clinique et Sante Communautaire, F es, Morroco); Netherlands: E. F. M. Wouters and G. J. Wesseling (Maastricht University Medical Center, Maastricht, the Netherlands); Nigeria: Daniel Obaseki (PI), Gregory Erhabor, Olayemi Awopeju, and Olufemi Adewole (Obafemi Awolowo University, Ile-Ife, Nigeria); Norway: Amund Gulsvik (PI), Tina Endresen, and Lene Svendsen (Department of Thoracic Medicine, Institute of Medicine, University of Bergen, Bergen, Norway); Pakistan: Asaad A. Nafees (PI), Muhammad Irfan, Zafar Fatmi, Aysha Zahidie, Natasha Shaukat, and Meesha Iqbal (Aga Khan University, Karachi, Pakistan); Philippines: Luisito F. Idolor (PI), Teresita S. de Guia, Norberto A. Francisco, Camilo C. Roa, Fernando G. Ayuyao, Cecil Z. Tady, Daniel T. Tan, Sylvia Banal-Yang, Vincent M. Balanag, Jr., Maria Teresita N. Reyes, and Renato. B. Dantes (Lung Centre of the Philippines, Philippine General Hospital, Nampicuan and Talugtug, the Philippines); Renato B. Dantes (PI), Lourdes Amarillo, Lakan U. Berratio, Lenora C. Fernandez, Norberto A. Francisco, Gerard S. Garcia, Teresita S. de Guia, Luisito F. Idolor, Sullian S. Naval, Thessa Reyes, Camilo C. Roa, Jr., Ma. Flordeliza Sanchez, and Leander P. Simpao (Philippine College of Chest Physicians, Manila, the Philippines); Poland: Ewa Nizankowska-Mogilnicka (PI), Jakub Frey, Rafal Harat, Filip Mejza, Pawel Nastalek, Andrzej Pajak, Wojciech Skucha, Andrzej Szczeklik, and Magda Twardowska, (Division of Pulmonary Diseases, Department of Medicine, Jagiellonian University School of Medicine, Krakow, Poland); Portugal: Cristina Barbara (PI), Fatima Rodrigues, Hermınia Dias, Joao Cardoso, João Almeida, Maria Joao Matos, Paula Simão, Moutinho Santos, and Reis Ferreira (the Portuguese Society of Pneumology, Lisbon, Portugal); Saudi Arabia: M. Al Ghobain (PI), H. Alorainy (PI), E. El-Hamad, M. Al Hajjaj, A. Hashi, R. Dela, R. Fanuncio, E. Doloriel, I. Marciano, and L. Safia (Saudi Thoracic Society, Riyadh, Saudi Arabia); South Africa: Eric Bateman (PI), Anamika Jithoo (PI), Desiree Adams, Edward Barnes, Jasper Freeman, Anton Hayes, Sipho Hlengwa, Christine Johannisen, Mariana Koopman, Innocentia Louw, Ina Ludick, Alta Olckers, Johanna Ryck, and Janita Storbeck, (University of Cape Town Lung Institute, Cape Town, South Africa); Sri Lanka: Kirthi Gunasekera (PI) and Rajitha Wickremasinghe (Medical Research Institute, Central Chest Clinic, Colombo, Sri Lanka); Sudan: Asma Elsony (PI), Hana A. Elsadig, Nada Bakery Osman, Bandar Salah Noory, Monjda Awad Mohamed, Hasab Alrasoul Akasha Ahmed Osman, Namarig Moham ed Elhassan, Abdel Mu‘is El Zain, Marwa Mohamed Mohamaden, Suhaiba Khalifa, Mahmoud Elhadi, Mohand Hassan, and Dalia Abdelmonam (the Epidemiological Laboratory, Khartoum, Sudan); Sweden: Christer Janson (PI), Inga Sif Olafsdottir, Katarina Nisser, Ulrike SpetzNystrom, Gunilla Hägg, and Gun-Marie Lund (Department of Medical Sciences: Respiratory Medicine and Allergology, Uppsala University, Uppsala, Sweden); Trinidad and Tobago: Terence Seemungal (PI), Fallon Lutchmansingh, and Liane Conyette (University of the West Indies, St. Augustine, Trinidad and Tobago); Tunisia: Imed Harrabi (PI), Myriam Denguezli, Zouhair Tabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, and Wahbi Belhaj Khalifa (University Hospital Farhat Hached, Faculte de M edecine, Sousse, Tunisia); Turkey: Ali Kocabas¸ (PI), Attila Hancioglu, Ismail Hanta, Sedat Kuleci, Ahmet Sinan Turkyilmaz, Sema Umut, and Turgay Unalan (Department of Chest Diseases, Cukurova University School of Medicine, Adana, Turkey); UK: Peter G. J. Burney (PI), Anamika Jithoo, Louisa Gnatiuc, Hadia Azar, Jaymini Patel, Caron Amor, James Potts, Michael Tumilty, Fiona McLean, and Risha Dudhaiya (National Heart and Lung Institute, Imperial College London, London, UK); United States: A. Sonia Buist (PI) (Oregon Health & Science University, Portland, Oregon); Mary Ann McBurnie, William M. Vollmer, and Suzanne Gillespie (Kaiser Permanente Center for Health Research, Portland, Oregon); Sean Sullivan (University of Washington, Seattle, Washington); Todd A. Lee and Kevin B. Weiss (Northwestern University, Chicago, Illinois); Robert L. Jensen and Robert Crapo (Latter Day Saints Hospital, Salt Lake City, Utah); Paul Enright (University of Arizona, Tucson, Arizona); David M. Mannino (PI), John Cain, Rebecca Copeland, Dana Hazen, and Jennifer Methvin (University of Kentucky, Lexington, Kentucky). Additional local support for BOLD clinical sites was provided by: Boehringer Ingelheim China (GuangZhou, China); Turkish Thoracic Society, BoehringerIngelheim, and Pfizer (Adana, Turkey); Altana, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Sharpe & Dohme, Novartis, Salzburger Gebietskrankenkasse and Salzburg Local Government (Salzburg, Austria); Research for International Tobacco Control, the International Development Research Centre, the South African Medical Research Council, the South African Thoracic Society GlaxoSmithKline Pulmonary Research Fellowship, and the University of Cape Town Lung Institute (Cape Town, South Africa); and Landspıtali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland, and AstraZeneca Iceland (Reykjavik, Iceland); GlaxoSmithKline Pharmaceuticals, Polpharma, Ivax Pharma Poland, AstraZeneca Pharma Poland, ZF Altana Pharma, Pliva Krakow, Adamed, Novartis Poland, Linde Gaz Polska, Lek Polska, Tarchominskie Zakłady Farmaceutyczne Polfa, Starostwo Proszowice, Skanska, Zasada, Agencja Mienia Wojskowego w Krakowie, Telekomunikacja Polska, Biernacki, Biogran, Amplus Bucki, Skrzydlewski, Sotwin, and Agroplon (Cracow, Poland); BoehringerIngelheim, and Pfizer Germany (Hannover, Germany); the Norwegian Ministry of Health's Foundation for Clinical Research, and Haukeland University Hospital's Medical Research Foundation for Thoracic Medicine (Bergen, Norway); AstraZeneca, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline (Vancouver, Canada); Marty Driesler Cancer Project (Lexington, Kentucky); Altana, Boehringer Ingelheim (Phil), GlaxoSmithKline, Pfizer, Philippine College of Chest Physicians, Philippine College of Physicians, and United Laboratories (Phil) (Manila, Philippines); Air Liquide Healthcare P/L, AstraZeneca P/L, Boehringer Ingelheim P/L, GlaxoSmithKline Australia P/L, Pfizer Australia P/L (Sydney, Australia), Department of Health Policy Research Programme, Clement Clarke International (London, UK); Boehringer Ingelheim and Pfizer (Lisbon, Portugal), Swedish Heart and Lung Foundation, The Swedish Association against Heart and Lung Diseases, Glaxo Smith Kline (Uppsala, Sweden), Seed Money Grant (PF20/0512), Aga Khan University, and Chiesi Pakistan (Pvt.) Limited (Karachi, Pakistan).