Pulmonary rehabilitation (PR) has many benefits for individuals with COPD. However, it is not clear whether PR could prevent the hazards of air pollution exposure. This study aimed to analyze the effects of biomass burning exposure on pulmonary inflammatory markers and pulmonary function in individuals with COPD, participants and non-participants of PR.
Methods35 subjects were divided into three groups: individuals with COPD who received PR (G1, n=15), those who did not (G2, n=10), and a control group composed of healthy individuals without COPD (CG, n=10). Measurements of lung function and concentrations of IL-6, IL-10, and TNF-α in exhaled breath condensate samples were collected. The assessment and concentrations of particulate matter (PM10), nitrogen dioxide (NO2), ozone (O3), temperature (T), and relative air humidity (RAH) were recorded in biomass burning and non-burning periods.
ResultsThere was a significant increase in the concentrations of air pollutants in the biomass burning period. In this period, an increase in IL-6 (G1p=0.041, G2 p=.012), and a reduction in the FEV1/FVC ratio (G1p=0.021, G2 p=.007) were observed in individuals with COPD. In G1, the increase in IL-6 concentrations correlated positively with O3 (r=0.693; p=.006), and negatively with RAH (r=−0.773; p=.003) in the burning period.
ConclusionsIndividuals with COPD exposed to biomass burning demonstrated increased pulmonary inflammation and a reduction in the FEV1/FVC ratio, regardless of their engagement in PR.
The main cause of Chronic Obstructive Pulmonary Disease (COPD) is smoking, including passive smoking.1 In underdeveloped countries, in addition to smoking, exposure to air pollution and biomass burning are risk factors for developing the disease2–5 and episodes of exacerbation.6 Despite technological advances, the impact of biomass burning in some regions is still a major problem for the health of the exposed population.7
The emission of high levels of air pollutants relates to an increase in respiratory symptoms, respiratory infections, and decreased lung function in COPD individuals as they are more vulnerable to additional stress on the respiratory tract caused by these aggressors.8 Inhalation of harmful gases and particulate matter can induce pulmonary inflammatory response orchestrated by inflammatory cells and pulmonary inflammatory mediators.9
The study of Guarnieri et al.10 performed with women exposed to smoke from biomass burning, observed an increase in cells and inflammatory markers such as interleukin (IL) -6, IL-8 and tumor necrosis factor (TNF-α), both at a pulmonary and systemic level.11
In addition to increased pulmonary and systemic inflammatory responses, exposure to biomass burning can cause respiratory symptoms such as coughing, dyspnea, and decreased lung function.12 This is evidenced in the study of Jin et al.13 in which there was a decrease in forced vital capacity (FVC) and forced expiratory flow (FEF50) in individuals with COPD after exposure to coal-burning.
In the face of these complications and impairment of the symptoms to which individuals with COPD are susceptible, physical training, as part of pulmonary rehabilitation, is widely known to have several beneficial effects on the health of these individuals,14 in addition to potential anti-inflammatory effects,15 however it is not known if physical training has this protective effect in individuals with COPD exposed to air pollutants.
Thus, the aim of this study was to analyze the effects of exposure to biomass burning on inflammatory markers and pulmonary function in individuals with COPD, participants and non-participants in a pulmonary rehabilitation program.
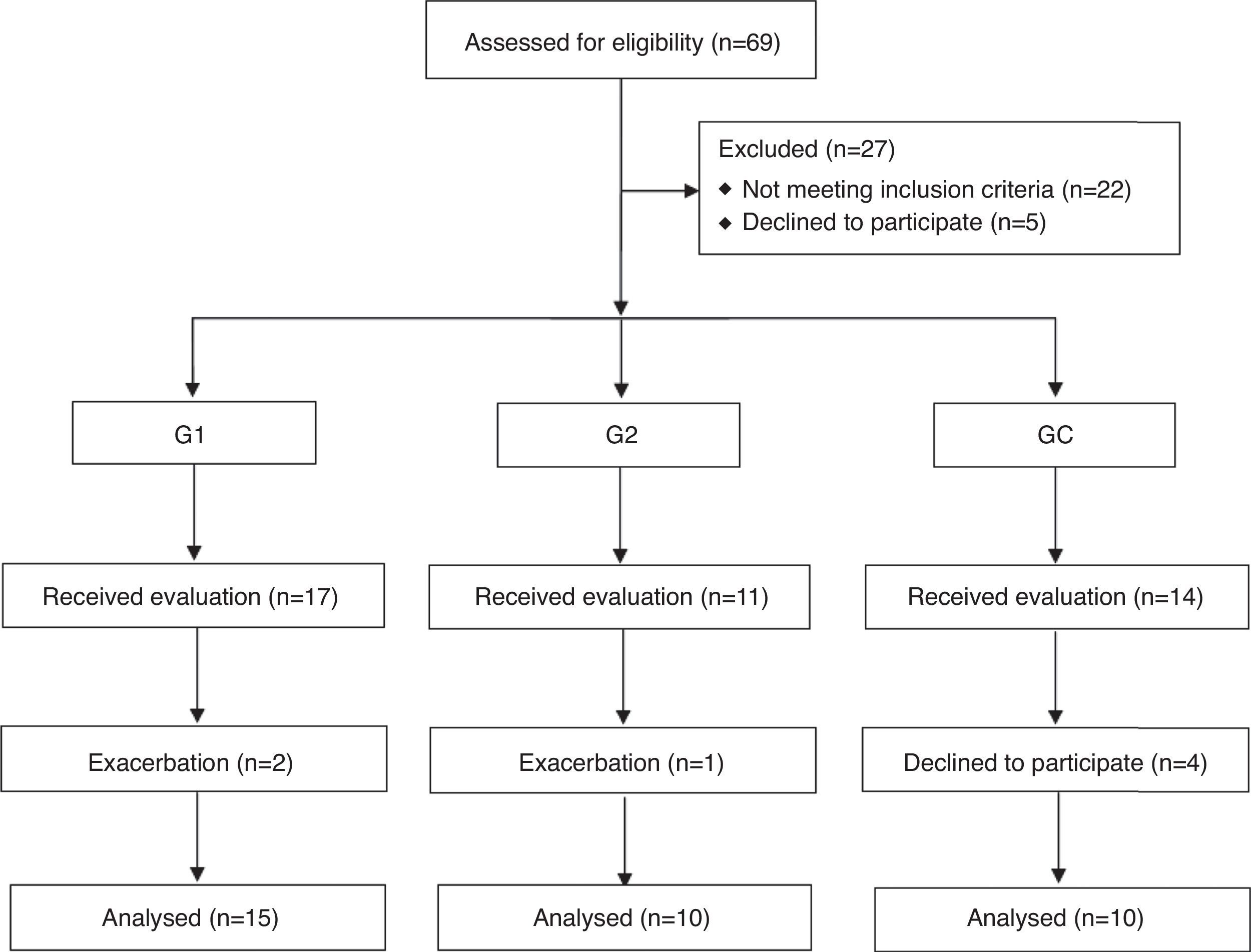
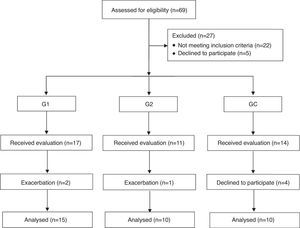
Material and methodsIn total, 69 subjects were recruited, however 22 did not meet the inclusion criteria and 5 refused to participate, thus, 42 individuals actually participated in the study. The subjects were divided into three groups, individuals with COPD who received pulmonary rehabilitation (G1, n=17), those who did not receive (G2; n=11), and a control group composed of healthy individuals without COPD (CG, n=14). From this sample, 3 and 4 exacerbated individuals dropped out prior to completion of the study, obtaining a final sample of 35 individuals (Fig. 1). Inclusion criteria were clinically stable individuals with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD),1 who had reported no changes in medications for at least 30 days, were not current smokers and were not taking oxygen therapy at home. For the control group, they had to have no pre-existing health condition that could influence pulmonary function and inflammatory process.
The G1 group received, in addition to pharmacological treatment, supervised monitoring of pulmonary rehabilitation twice weekly, each session lasting 60minutes, consisting of aerobic and resistance components. Aerobic component was performed on a treadmill, and the walking speed was set at 60% of the average speed obtained from the six minute walk test (6MWT). Treadmill speed was increased until the participants reached 115% of their initial average speed from the 6MWT at the end of the protocol. The resistance component was carried out on a conventional weight machine. G1 group started at 60% of their one-repetition maximum (1-RM). They performed three sets of ten repetitions for each exercise. The load was increased by 4% every four sessions until they reached 80% of their initial 1-RM at the end of protocol. The G2 group received only pharmacological treatment.
The study was conducted at the Center for Studies and Assistance in Physiotherapy and Rehabilitation, Faculty of Science and Technology – São Paulo State University of Presidente Prudente/SP, Brazil.
Individuals signed the free and informed consent in accordance with the Declaration of Helsinki of the World Medical Association. The study was approved by the Research Ethics Committee of the Faculty of Science and Technology – UNESP, PresidentePrudente/SP (No. 5402/13).
The evaluations were performed in the period of biomass burning and non-burning. Individuals performed two nonconsecutive days of assessments. On the first day an interview was held to obtain personal data; smoking history (smoking years, cigarettes/day, pack-years, and years of abstinence) and comorbidities. To attest to smoking abstinence carbon monoxide concentration in exhaled air (COex) was verified, followed by lung function. On the second day the measurement of lung inflammatory markers through exhaled breath condensate (EBC) was performed.
The particulate matter concentrations (PM10), nitrogen dioxide (NO2) and ozone (O3); temperature (T) and relative air humidity (RAH) were obtained through daily active sampling by the automatic weather station of the State of São Paulo located in PresidentePrudente/SP. Data on environmental pollution, T, and RAH were also recorded during the periods of biomass burning and non-burning.
COex and carboxyhemoglobin (HbCO) were verified using a carbon monoxide analyzer (Micro CO meter, Cardinal Health, UK).16 The concentration of COex was expressed in parts per million (ppm) and HbCO. Spirometry was performed according to the recommendations of the ATS/ERS, using a portable spirometer (Spirobank-MIR®, Italy, version 3.6).17 The values related to the Brazilian population were used.18
Exercise capacity was assessed by 6MWT according to the recommendations of the ATS.19 The 6MWT was only performed on the group of COPD that participated in the pulmonary rehabilitation program, once the remaining two groups did not receive physical intervention.
A specific enzyme immunoassay kit ELISA (Ready-SET-Go, eBioscience, Affymetrix, California, USA) was used to measure IL-6, IL-10 and TNF-α concentrations in the EBC.20 EBC is a promising noninvasive tool that can be useful to evaluate biomarkers in lung disease.21 EBC was collected using a glass condenser tube that was mounted in polystyrene boxes filled with crushed ice and salt crystals. The temperature in the device was about −15°C. A mouth adaptor was added to the circuit, to aid the individuals to breathe tidally for 10minutes while wearing a nose clip.22 EBC samples were stored in polypropylene tubes at −70°C until analysis. The assay was validated directly by gas chromatography/mass spectrometry. The intra-assay and inter-assay variability were ≤7%.
Statistical analysisData were expressed as mean and standard deviation, median and interquartile range (25–75%). The normality of the data was assessed by the Shapiro–Wilk test. For intragroup comparison between periods of biomass burning and non-burning, the Student's t test or Wilcoxon test was used, depending on the normality of the data. For intergroup comparisons, ANOVA with Bonferroni post test or the Kruskal–Wallis test with Dunn's post test were performed according to data homogeneity (Levene's test) comparing the delta values (Δ=burning−non-burning). For correlations, the Pearson or Spearman tests were used according to the normality of the data. From a significant correlation, linear regression was performed from 10 logarithms of each variable, which were adjusted for age, body mass index, and pack-years. The statistical software used was SPSS 15.0 and values of p<.05 were considered significant.
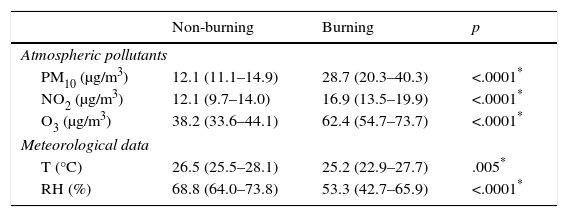
ResultsDuring the burning of biomass the concentration of air pollutants was significantly higher compared to the period of non-biomass burning. The Temperature and Relative Air Humidity were lower in the period of burning. Table 1 shows the concentrations of air pollutants and meteorological variables in the city of Presidente Prudente/SP in the non-burning and burning periods.
Atmospheric pollutants and meteorological data in urban area, non-burning and burning periods.
| Non-burning | Burning | p | |
|---|---|---|---|
| Atmospheric pollutants | |||
| PM10 (μg/m3) | 12.1 (11.1–14.9) | 28.7 (20.3–40.3) | <.0001* |
| NO2 (μg/m3) | 12.1 (9.7–14.0) | 16.9 (13.5–19.9) | <.0001* |
| O3 (μg/m3) | 38.2 (33.6–44.1) | 62.4 (54.7–73.7) | <.0001* |
| Meteorological data | |||
| T (°C) | 26.5 (25.5–28.1) | 25.2 (22.9–27.7) | .005* |
| RH (%) | 68.8 (64.0–73.8) | 53.3 (42.7–65.9) | <.0001* |
Data are expressed as medians (interquartile range).
PM10: particulate matter 10μm; NO2: nitrogen dioxide; O3: ozone; T: temperature; UR: atmospheric relative humidity.
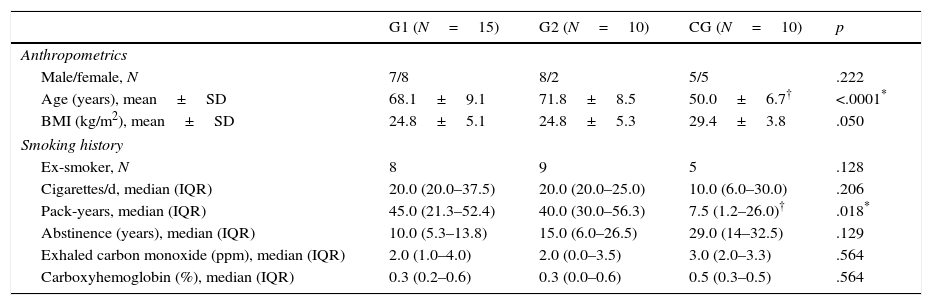
Table 2 presents the characteristics of the groups. Both groups with COPD presented higher smoking load, represented by pack-years, compared to the control group. There was an increase in IL-6 concentrations in both groups with COPD in the burning period compared to the non-burning period. There was no significant difference in the intergroup comparison (Table 3).
Characteristics of participants.
| G1 (N=15) | G2 (N=10) | CG (N=10) | p | |
|---|---|---|---|---|
| Anthropometrics | ||||
| Male/female, N | 7/8 | 8/2 | 5/5 | .222 |
| Age (years), mean±SD | 68.1±9.1 | 71.8±8.5 | 50.0±6.7† | <.0001* |
| BMI (kg/m2), mean±SD | 24.8±5.1 | 24.8±5.3 | 29.4±3.8 | .050 |
| Smoking history | ||||
| Ex-smoker, N | 8 | 9 | 5 | .128 |
| Cigarettes/d, median (IQR) | 20.0 (20.0–37.5) | 20.0 (20.0–25.0) | 10.0 (6.0–30.0) | .206 |
| Pack-years, median (IQR) | 45.0 (21.3–52.4) | 40.0 (30.0–56.3) | 7.5 (1.2–26.0)† | .018* |
| Abstinence (years), median (IQR) | 10.0 (5.3–13.8) | 15.0 (6.0–26.5) | 29.0 (14–32.5) | .129 |
| Exhaled carbon monoxide (ppm), median (IQR) | 2.0 (1.0–4.0) | 2.0 (0.0–3.5) | 3.0 (2.0–3.3) | .564 |
| Carboxyhemoglobin (%), median (IQR) | 0.3 (0.2–0.6) | 0.3 (0.0–0.6) | 0.5 (0.3–0.5) | .564 |
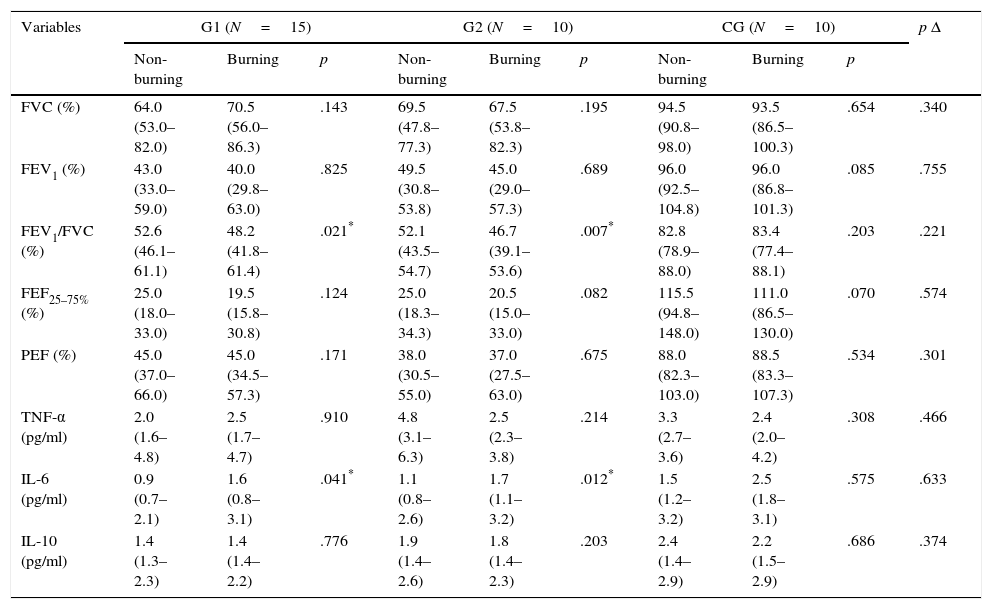
Comparison of spirometric values, interleukins, and delta (Δ) differences between the groups during the non-burning and burning periods.
| Variables | G1 (N=15) | G2 (N=10) | CG (N=10) | p Δ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-burning | Burning | p | Non-burning | Burning | p | Non-burning | Burning | p | ||
| FVC (%) | 64.0 (53.0–82.0) | 70.5 (56.0–86.3) | .143 | 69.5 (47.8–77.3) | 67.5 (53.8–82.3) | .195 | 94.5 (90.8–98.0) | 93.5 (86.5–100.3) | .654 | .340 |
| FEV1 (%) | 43.0 (33.0–59.0) | 40.0 (29.8–63.0) | .825 | 49.5 (30.8–53.8) | 45.0 (29.0–57.3) | .689 | 96.0 (92.5–104.8) | 96.0 (86.8–101.3) | .085 | .755 |
| FEV1/FVC (%) | 52.6 (46.1–61.1) | 48.2 (41.8–61.4) | .021* | 52.1 (43.5–54.7) | 46.7 (39.1–53.6) | .007* | 82.8 (78.9–88.0) | 83.4 (77.4–88.1) | .203 | .221 |
| FEF25–75% (%) | 25.0 (18.0–33.0) | 19.5 (15.8–30.8) | .124 | 25.0 (18.3–34.3) | 20.5 (15.0–33.0) | .082 | 115.5 (94.8–148.0) | 111.0 (86.5–130.0) | .070 | .574 |
| PEF (%) | 45.0 (37.0–66.0) | 45.0 (34.5–57.3) | .171 | 38.0 (30.5–55.0) | 37.0 (27.5–63.0) | .675 | 88.0 (82.3–103.0) | 88.5 (83.3–107.3) | .534 | .301 |
| TNF-α (pg/ml) | 2.0 (1.6–4.8) | 2.5 (1.7–4.7) | .910 | 4.8 (3.1–6.3) | 2.5 (2.3–3.8) | .214 | 3.3 (2.7–3.6) | 2.4 (2.0–4.2) | .308 | .466 |
| IL-6 (pg/ml) | 0.9 (0.7–2.1) | 1.6 (0.8–3.1) | .041* | 1.1 (0.8–2.6) | 1.7 (1.1–3.2) | .012* | 1.5 (1.2–3.2) | 2.5 (1.8–3.1) | .575 | .633 |
| IL-10 (pg/ml) | 1.4 (1.3–2.3) | 1.4 (1.4–2.2) | .776 | 1.9 (1.4–2.6) | 1.8 (1.4–2.3) | .203 | 2.4 (1.4–2.9) | 2.2 (1.5–2.9) | .686 | .374 |
Data are expressed as medians (interquartile range).
p Δ: difference between the deltas; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; FEV1/FVC: ratio of FEV1 and FVC; FEF25–75%: expiratory flow between 25% and 75% of FVC; PEF: peak expiratory flow; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-10: interleukin 10.
In G1, the increase in IL-6 levels correlated positively with O3 (r=0.693; p=.006), and negatively with RAH (r=−0.773; p=.003) during the burning period. In the unadjusted linear regression it was observed that for each increase of 1g/m3 of O3 there was an increase of 0.691pg/ml of IL-6 in the burning period (β: 0.691; CI: 0.972–4.707). However, after adjustment for confounders such as body mass index, age, and pack-years the relationship between O3 and IL-6 was no longer significant (β: −0.005; CI: −0.014 to 0.005).
There was a significant decrease in the FEV1/FVC ratio in individuals of both groups with COPD in the burning period compared to the non-burning period. There was no difference in the intergroup comparison (Table 3). Moreover, in relation to functional capacity (6MWT), G1 presented a significant improvement during burning period compared to the non-burning (504.1±67.2 vs 465.1±68.4 meters; p=.005).
DiscussionThis study showed a significant increase in the concentrations of air pollutants in the period of biomass burning compared to the period of non-biomass burning. Individuals with COPD who received pulmonary rehabilitation (G1) showed an increase in functional capacity, with an improved performance in the 6MWT during the burning period. Moreover, there was an increase in IL-6 expression and a reduction in the FEV1/FVC ratio in the period of biomass burning. In the same way, individuals with COPD who did not received pulmonary rehabilitation (G2) also presented increase in IL-6 expression and a reduction in the FEV1/FVC ratio. In the control group no difference was observed.
IL-6 has been identified as an important mediator of the inflammatory process in chronic lung diseases.23 Bucchione et al.24 suggest that the increase in IL-6 concentration in the lungs of COPD patients may indicate inflammation of the airways. IL-6 production is carried out not only by the innate immune system cells (P. Ex. Macrophages, dendritic cells, mast cells, and neutrophils), but also by lung epithelial cells. Thus, since the epithelium of the respiratory tract is the first pathway of contact between the body and the external environment, epithelial cells and alveolar macrophages may play a role in signaling and IL-6 release in response to environmental changes.25
In this study there was an increase in inflammatory markers in the lungs in both groups of individuals with COPD during the biomass burning and a positive correlation with the concentration of O3. Our findings corroborate the study of Dameras et al.,26 which showed that exposure to O3 influences the modulation of IL-6 release in the airway epithelium. Besides measuring inflammation, the increase in IL-6 observed with exposure to O3 in individuals with COPD may indicate lung epithelial injury.23
This type of epithelial damage, added to modifications in the cellular profile and function, has been identified as one of the contributing factors for the development of COPD.26,27 In addition, IL-6 has been identified as a biomarker to predict mortality in COPD.28
In this study, the analysis of multivariate models adjusted for variable pack-years showed a loss in the cause and effect relationship between O3 and IL-6, suggesting that the correlation observed in the present study was influenced by the smoking load of individuals with COPD.
It is possible that this explains the fact that there was a correlation between O3 and IL-6 only in the group with higher values of pack-years. This finding is in agreement with the study of Moraes et al.29 that reported an increase in IL-6 observed in individuals with COPD which may be associated with smoking history, particularly in those with a smoking load of more than 30 pack-years.
In patients with stable COPD, lung inflammation seems to persist regardless of smoking cessation, a factor which may contribute to the increased decline in lung function.30,31 Previous studies have shown that lung function is an important marker of the effects of air pollution in the exposed population.8
In this study, there was a decrease in the FEV1/FVC ratio in individuals with COPD in the biomass burning period. Other studies have also reported a reduction in this parameter after exposure to biomass burning.32,33
The alterations observed in the present study in relation to the inflammatory status and lung function occurred independently of whether the individual with COPD performed or did not perform supervised physical training, suggesting that physical training may not be able to mitigate the harmful effects of exposure to air pollution.34 This may be because individuals with COPD are particularly vulnerable to stress in the airways caused by exposure to pollutants.
Exposure to air pollution is associated with increased morbidity in these patients, including increased respiratory symptoms, inflammation, and decreased lung function, it is the frequent cause of exacerbations, causing visits to emergency services or hospitalization.35
In a recent study of Fisher at al. (2016) there was a negative association between doing physical activity (sports and cycling) and hospitalizations of patients with COPD, and a positive association between exposure to NO2 and the hospitalizations of these patients, but the two events are independent from each other.36 Our results showed that pulmonary rehabilitation was able to improve the functional capacity of the individuals with COPD, however, their engagement in pulmonary rehabilitation did not prevent the hazards of biomass burning exposure to the respiratory tract of these individuals.
ConclusionsIt is concluded that individuals with COPD exposed to biomass burning presented increased lung inflammation and a reduction in the FEV1/FVC ratio independently of participation in a pulmonary rehabilitation program.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial supportGrant #2012/12901-0, São Paulo Research Foundation (FAPESP). FAPESP had no role in study design, data collections and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestNone declared.
The authors would like to acknowledge the financial support given by São Paulo Research Foundation (FAPESP).