Transbronchial lung cryobiopsy (TBLC) is increasingly used in the diagnosis of diffuse lung disease (DLD), but no data have yet been published on the learning curve associated with this technique.
AimTo evaluate diagnostic yield, lung tissue sample length and area, and procedure-related complications in a cohort of TBLC procedures to define the learning curve and threshold for proficiency.
MethodsRetrospective analysis of the first 100 TBLCs performed in different segments of the same lobe in patients with suspected DLD. We compared diagnostic yield, sample length and area, and complications between consecutive groups of patients.
ResultsThe overall diagnostic yield for TBLC was 82%. Median sample length was 5.4mm (IQR, 5–6) and median area was 19.5mm2 (IQR, 13.3–25). Pneumothorax was the most common complication (18%). On comparing the two groups of 50 consecutive patients, a significant difference was found for diagnostic yield (74% vs 90%; p=0.04), sample length (5.0mm [2.5–16] vs 6.0mm [4–12;] p<0.01) and area (17.5mm2 [6–42] vs 21.5mm2 [10–49]; p<0.01). Logarithm regression was applied to median diagnostic yield and sample length and area for groups of 10 consecutive patients to define the learning curve, which plateaued after approximately 70 procedures.
ConclusionsOur findings suggest that proficiency in TBLC is achieved at approximately the 70th procedure; however they need to be validated in more series and cohorts.
Diffuse lung diseases (DLD) comprise a broad variety of disorders. Accurate diagnosis is, therefore crucial, since the various entities within DLD have different prognoses and therapeutic approaches, particularly since the advent of antifibrotic therapy.1–3 Current guidelines recommend multidisciplinary diagnosis (MDD) as the gold standard.1,2,4–6 Histology is a necessary diagnostic tool when clinical, radiological, and bronchoalveolar lavage findings are inconclusive.1,2,4–6 In such cases, surgical lung biopsy (SLB) is considered the gold standard for tissue sampling.4–6 This procedure, however, is costly and has risks, hence the continued search for other diagnostic options. One possibility that has attracted considerable attention recently is transbronchial lung cryobiopsy (TBLC).7–10
Endobronchial cryotherapy is a well-established endoscopic technique for therapeutic purposes, namely the palliative treatment of obstructing endobronchial tumors.11,12 Its potential usefulness in transbronchial biopsy was first described by Babiak et al.8 in 2009. Since then, several authors have shown the feasibility of using TBLC for obtaining adequate fragments of lung parenchyma.9,10 Cryobiopsy fragments were larger than those obtained by conventional transbronchial biopsy, did not contain crush artifacts, and in addition, there were few complications. High overall diagnostic yields, typically above 80%, have been reported.10,13–15 Tomassetti et al.16 showed that TBLC had a similar diagnostic accuracy to SLB in the multidisciplinary diagnosis of idiopathic lung fibrosis (IPF), which is certainly the most challenging topic in DLD diagnosis. Pneumothorax is the main complication of TBLC, although studies have reported variable incidence rates (0–25.9%, mean, 8.8%).13,14
Although high diagnostic yields have generally been reported for TBLC, none of the studies to date have analyzed or discussed the learning curve required to achieve proficiency in this endoscopic diagnostic technique. While specific approaches may differ in terms of patient sedation, bronchoscopic procedure, and bronchoscopist (interindividual variability), as with all bronchoscopic interventions, there is a threshold of procedures needed to acquire the sufficient experience to perform TBLC adequately and efficiently.17–20
The aim of this study was to evaluate the learning curve for the performance of TBLC in terms of diagnostic yield, length and area of the lung tissue samples obtained, and associated complications.
Materials and methodsPatientsThis retrospective analysis included patients who had undergone TBLC in the bronchoscopy unit of the pulmonology department at Centro Hospitalar São João, in Porto, Portugal in the context of a DLD diagnostic work-up. All the patients were evaluated at the DLD outpatient clinic. A detailed history including drug and occupational exposure was taken and all the patients underwent a complete physical examination in addition to complete blood count, serum biochemistry and coagulation tests, lung function tests and arterial blood gases, thoracic high resolution computed tomography (HRCT), an electrocardiogram, and an echocardiogram. Exclusion criteria included the usual contraindications for TBLC10: platelet count <70,000/mm3, international normalized ratio >1.5 or activated partial thromboplastin time >50s, partial pressure of oxygen in arterial blood (PaO2) <55mmHg, forced expiratory capacity (FVC) <50%, forced expiratory volume in the first second (FEV1) <0.8L, diffusing capacity for carbon monoxide (DLCO) <35%, diffuse bullous disease, heart disease, or a transthoracic echocardiogram estimated pulmonary artery systolic pressure >40mmHg.10 Written informed consent was obtained from all patients. The study was approved by Centro Hospitalar de São João's ethical committee.
ProcedureThe TBLC procedure was performed using a combination of rigid bronchoscopy (tracheoscope 14mm, Karl Storz, Germany) and flexible bronchoscopy (Olympus BF-XT40, Europe) under general anesthesia with manual jet ventilation (working pressure of approximately 2bar). A flexible cryoprobe (90cm with a 2.4mm diameter; Erbokryo, Erbe, Germany) was introduced through the working channel of the flexible bronchoscope. Biopsies were taken under fluoroscopic guidance from an optimal distance between the probe and the thoracic wall of 10–20mm. Biopsy sites were selected based on HRCT abnormalities evaluated in DLD multidisciplinary team meeting (MDD).
Once brought into position, the probe was cooled to −85°C with nitrogen oxide for approximately 5–6s (3–4s when sarcoidosis was the main suspicion), thus freezing the lung tissue in contact with the probe. The frozen specimen attached to the tip of the probe was removed by pulling out the cryoprobe together with the bronchoscope. In all procedures, a Fogarty balloon was prophylactically placed in the selected segmental bronchus and inflated immediately after the biopsy. When bleeding occurred, the Fogarty balloon was deflated only after cessation of bleeding and before any additional biopsies were performed. Biopsy specimens were taken from different segments of one lobe. The samples, still attached to the probe, were first inserted in saline and then in formalin (Fig. 1). Following the procedure, patients were extubated and kept under observation. After 3h, a chest X-ray was performed to exclude pneumothorax and the patient was discharged. Complications such as iatrogenic pneumothorax, endobronchial bleeding, and 30-day post-interventional morbidity and mortality were documented. Pneumothorax was described according to observation measures or chest tube insertion requirements. Endobronchial bleeding was classified using the British Thoracic Society (BTS) system: mild bleeding-continued suctioning of blood from the airways, bleeding stops spontaneously; moderate bleeding-intubation of the biopsied segment with the bronchoscope in the wedge position, use of adrenaline or cold saline to stop bleeding; severe bleeding-placement of bronchus blocker or catheter, applying fibrin sealant, resuscitation, blood transfusion, admission to critical care unit or death.21
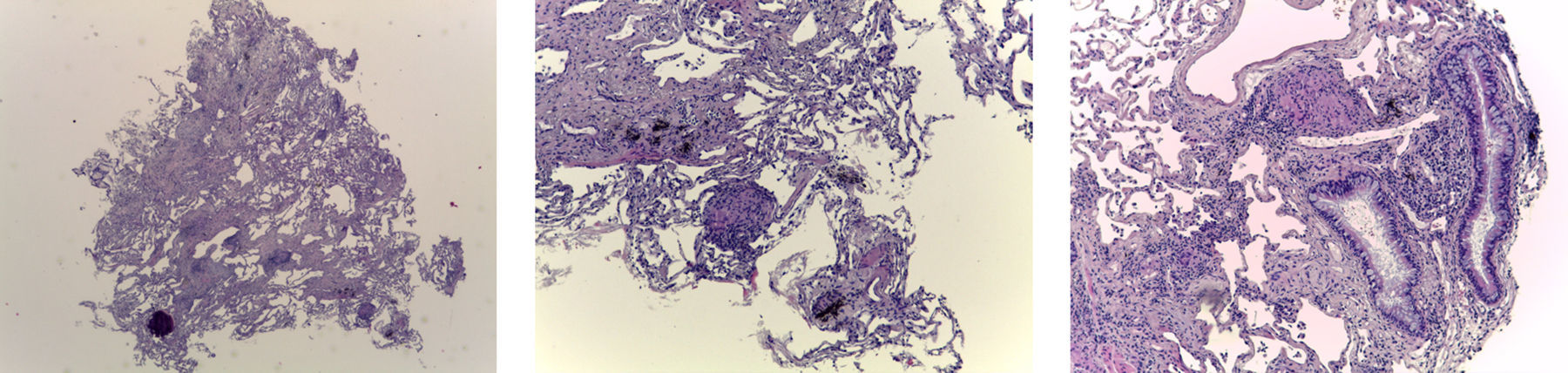
Pathologic assessmentThe sample tissue was formalin-fixed for at least 6h and at most 24h prior to paraffin embedding. Sections measuring 3μm were stained with hematoxylin–eosin. In all cases serial cuts were made at three levels, with additional use of special stains or immunohistochemical stains where necessary. All samples were measured under the optical microscope at 40× magnification and the area of each fragment evaluated. The presence of pleural tissue was recorded. The artifact areas were included in the measurement, however when the fragments were only pleura or adipose tissue they were not measured.
The biopsy was considered adequate if at least one of the fragments consisted of alveolated lung parenchyma (Fig. 2). The diagnosis was made based on the same criteria used for surgical biopsy.
Diagnostic criteriaThe TBLC was considered to be diagnostic when the sample analysis showed histologic features compatible with a particular histologic pattern that was determinant for a final diagnosis. A possible diagnosis in MDD analysis associated with a low confidence histology was not considered.
StatisticsDescriptive statistics are presented as frequency, percentage, mean, median, quartiles, and minimum and maximum values. The Chi-square test was used to assess differences between frequencies in groups with and without diagnosis and between groups of consecutive patients (two groups of 50, four groups of 25, and 10 groups of 10). The Mann–Whitney U test and Kruskal–Wallis test were used to assess median values for continuous variables. Diagnostic yield (defined as percentage of procedures for which TBLC provided a definitive diagnosis) and sample length and area were used as measures of TBLC performance. To plot the corresponding learning curves we fitted logarithmic regressions for the relationship between median values for these three variables in the 10 subgroups of 10 patients. p values <0.05 were considered to be statistically significant. All data were analyzed in RStudio, an environment for R language.
ResultsDiagnostic yield and complicationsBetween May 2014 and December 2016, 154 patients underwent TBLC as part of a MDD approach for DLD. Of these, 54 were excluded from our study as the biopsies had been performed in two lobes. In the remaining 100 patients, biopsy specimens had been taken from different segments of the same lobe. The mean (SD) age of the patients was 57.15 (13.02) years and 64% were male. Median FVC was 90% (interquartile range [IQR], 75.6–108.3), median DLCO was 58.8% (IQR, 46.8–75), and median PaO2 was 81.5mmHg (IQR, 74.6–88.3). A median of three TBLCs (IQR, 3–4) were performed per patient and the lower right lobe was chosen in 61% of cases. All the samples were considered adequate. Median sample length was 5.4mm (IQR, 5–6) and the median area was 19.5mm2 (IQR, 13.3–25) (Table 1). Samples from 40 patients contained pleura. A diagnosis was established in 82 patients (diagnostic yield, 82%). In 25 patients, identification of TBLC features led to a change in the main initial diagnosis. In the absence of TBLC, SLB would certainly have been considered in 68 of these 82 patients, since the remaining 14 patients were either not in a suitable clinical condition to be submitted to SLB or refused this approach when diagnostic procedures were discussed.
Demographic and clinical characteristics of patients who underwent transbronchial lung cryobiopsy.
| n | % | Median | IQR | |
|---|---|---|---|---|
| Age | 100 | 60.5 | 50.0–67.0 | |
| Sex | ||||
| Male | 64 | 64.0 | ||
| Female | 36 | 36.0 | ||
| FVC (% predicted) | 89 | 90.0 | 75.6–108.3 | |
| DLCO (% predicted) | 77 | 58.8 | 46.8–75.0 | |
| PaO2(mmHg) | 56 | 81.5 | 74.6–88.3 | |
| Length of samples (mm) | 100 | 5.4 | 5.0–6.0 | |
| Area of samples (mm3) | 90 | 19.5 | 13.3–25.0 | |
| Biopsy location | ||||
| RLL | 61 | 61.0 | ||
| LLL | 18 | 18.0 | ||
| RUL | 12 | 12.0 | ||
| LUL | 9 | 9.0 | ||
| Complications | ||||
| Pneumothorax | 18 | 18.0 | ||
| Bleeding | 3 | 3.0 | ||
| Histological patterns | ||||
| Hypersensitivity pneumonitis | 23 | 23.0 | ||
| Sarcoidosis | 17 | 17.0 | ||
| Smoking-related diseasesa | 16 | 16.0 | ||
| UIP/IPF | 9 | 9.0 | ||
| UIP/CTD-ILD | 1 | 1.0 | ||
| OP | 1 | 1.0 | ||
| NSIP | 4 | 4.0 | ||
| Otherb | 11 | 11.0 | ||
| Without diagnosis | 18 | 18.0 | ||
FVC – forced vital capacity; DLCO – diffusing capacity for carbon monoxide; PaO2 – oxygen arterial pressure; RLL – right lower lobe; LLL – left lower lobe; RUL – right upper lobe; LUL – left upper lobe; UIP – usual interstitial pneumonia; IPF – idiopathic pulmonary fibrosis; CTD-ILD – connective tissue disease-related interstitial lung disease; NSIP – non-specific interstitial pneumonia.
The most frequent diagnoses were hypersensitivity pneumonitis (23 patients, including seven with UIP-like features and one with nonspecific interstitial pneumonia-like features); sarcoidosis (17 patients); and smoking-related disorders (16 patients, including 10 with desquamative interstitial pneumonia, three with respiratory bronchiolitis associated with interstitial pneumonia, and three with smoking-related interstitial fibrosis) (Table 1). Pneumothorax was the most common complication, reported for 18 patients (Table 1). Of these, 13 required drainage (median time of 2.3 days) and just one had a persistent air leak managed only with chest tube drainage. Nine patients with pneumothorax had pleura in their biopsy samples (50%), compared with 31 of the 82 patients without pneumothorax (37.8%, nonsignificant difference). One patient had a pneumomediastinum. There were several cases of mild bleeding, but these were not documented as we considered them to be standard events for this procedure. Three patients had moderate bleeding and there were no cases of serious bleeding. No deaths occurred during TBLC, but one patient, diagnosed with IPF following the TBLC, died 20 days after the procedure. The patient had rapid-progressing disease and was already exhibiting clinical and functional decline (PaO2, 58mmHg) at the time of the TBLC. Despite antifibrotic treatment, the clinical deterioration persisted. There were no signs of acute exacerbation (i.e., no images of consolidation or ground glass opacities on HRCT, or even histologic signs of diffuse alveolar damage), and death was attributed to disease progression, independently of the TBLC.
No significant differences were found between the baseline characteristics of patients who were diagnosed following TBLC and those who were not. Right lower lobe was the most frequent biopsy location, both for patients with a definite diagnosis of TBLC (58.02%) and those without (72.22%). There were also no significant differences between these groups for number of biopsies or sample length (5.5mm vs 4.5mm) or area (20.0mm2 vs 14.8mm2), although samples were generally larger in patients with a diagnosis. Since a shorter freezing time (3–4s) was used when there was a strong suspicion of sarcoidosis, the area of the samples was significantly smaller in these cases (median 14.5mm2 [IQR, 6.0–20.0] and mean 15.91mm2 [IQR, 6.0–25.0] compared with median 20.2mm2 [IQR, 16.25–26.0] and mean 21.22mm2 [6.2–49] for samples obtained using a longer freezing time; p=0.022).
The rate of complications was also similar between these two subgroups. Pneumothorax was the most common complication, and was reported in 18.3% of patients with a diagnosis and 16.7% of those without.
The learning curveNo significant differences were observed for median FVC, DLCO, or PaO2 between the two subgroups of 50 consecutive patients (Table 2) or the four groups of 25 consecutive patients (Table 3).
Distribution of variables in two groups of 50 consecutive patients who underwent transbronchial lung cryobiopsy.
| Variable | First group of 50 patients | Second group of 50 patients | p value* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Mean | Median | Min | Max | n | % | Mean | Median | Min | Max | ||
| FVC (%) | 44 | 92.48 | 90.9 | 52.7 | 142.4 | 45 | 91.83 | 87.9 | 47.6 | 153.0 | 0.65 | ||
| DLCO | 38 | 60.23 | 60.8 | 25.9 | 98.5 | 39 | 62.21 | 53.2 | 26.0 | 107.7 | 0.88 | ||
| PaO2(mmHg) | 31 | 82.12 | 84.0 | 67.0 | 97.0 | 25 | 81.43 | 79.0 | 63.9 | 108.0 | 0.61 | ||
| No. of biopsies | 50 | 3.0 | 3.0 | 1.0 | 4.0 | 49 | 3.1 | 3.0 | 1.0 | 4.0 | 0.68 | ||
| Length of samples (mm) | 50 | 5.2 | 5.0 | 2.5 | 16.0 | 50 | 5.9 | 6.0 | 4.0 | 12.0 | <0.01 | ||
| Area (mm3) | 40 | 17.5 | 17.5 | 6.0 | 42.0 | 50 | 22.5 | 21.5 | 10.0 | 49.0 | <0.01 | ||
| Diagnostic yield (%) | 37 | 74.0 | 45 | 90.0 | 0.04 | ||||||||
| Complications (no.) | |||||||||||||
| Pneumothorax | 12 | 24.0 | 6 | 12.0 | 0.12 | ||||||||
| Bleeding | 1 | 2.0 | 2 | 4.0 | |||||||||
Distribution of variables in four groups of 25 consecutive patients who underwent transbronchial lung cryobiopsy.
| Variable | First group of 25 patients | Second group of 25 patients | Third group of 25 patients | Fourth group of 25 patients | p value* | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Mean | Median | Min | Max | n | % | Mean | Median | Min | Max | n | % | Mean | Median | Min | Max | n | % | Mean | Median | Min | Max | ||
| FVC | 20 | 91.4 | 90.6 | 52.7 | 142.4 | 24 | 93.4 | 93.3 | 58.5 | 123.0 | 24 | 86.3 | 85.0 | 47.6 | 129.2 | 21 | 98.13 | 95.0 | 53.5 | 153.0 | 0.38 | ||||
| DLCO | 17 | 61.5 | 60.8 | 25.9 | 98.5 | 21 | 59.2 | 56.9 | 27.1 | 87.2 | 21 | 60.0 | 53.2 | 26.0 | 101.0 | 18 | 64.79 | 57.3 | 38.8 | 107.7 | 0.94 | ||||
| PaO2(mmHg) | 17 | 81.3 | 82.0 | 67.0 | 93.2 | 14 | 83.2 | 86.6 | 67.9 | 97.0 | 13 | 83.9 | 80.2 | 64.6 | 108.0 | 12 | 78.75 | 78.3 | 63.9 | 89.0 | 0.56 | ||||
| No. of biopsies | 25 | 3.2 | 4.0 | 1.0 | 4.0 | 25 | 2.8 | 3.0 | 1.0 | 4.0 | 25 | 3.0 | 3.0 | 1.0 | 4.0 | 24 | 3.3 | 3.00 | 2.0 | 4.0 | 0.10 | ||||
| Length of samples (mm) | 25 | 5.0 | 5.0 | 3.0 | 9.0 | 25 | 5.4 | 5.0 | 2.5 | 16.0 | 25 | 5.7 | 5.6 | 4.0 | 7.5 | 25 | 6.2 | 6.0 | 4.0 | 12.0 | 0.01 | ||||
| Area of samples (mm3) | 21 | 17.6 | 17.5 | 6.2 | 42.0 | 19 | 17.5 | 15.0 | 6.0 | 35.0 | 25 | 21.4 | 20.0 | 10.0 | 41.2 | 25 | 23.5 | 23.5 | 11.0 | 49.0 | 0.01 | ||||
| Diagnostic yield | 16 | 64.0 | 21 | 84.0 | 22 | 88.0 | 23 | 92.0 | 0.01 | ||||||||||||||||
| Complications | |||||||||||||||||||||||||
| Pneumothorax | 6 | 24.0 | 6 | 24.0 | 5 | 20.0 | 1 | 4.0 | 0.06 | ||||||||||||||||
| Bleeding | 1 | 4.0 | 0 | 0.0 | 2 | 8.0 | 0 | 0.0 | |||||||||||||||||
A significant difference was found for diagnostic yield between the subgroups of 50 patients (74% vs 90%; p=0.04) (Table 2) and 25 patients. In this second case, there was a clear increase in yield over the four periods (64% for group 1, 84% for group 2, 88% for group 3, and 92% for group 4; p<0.01) (Table 3).
Median sample length and area were significantly greater (p<0.01) in the second group of 50 patients (5.0mm [IQR, 2.5–16] and 17.5mm2 [IRQ, 6–42] respectively) compared with the first group (6.0mm [IQR, 4–12] and 21.5mm2 [IQR, 10–49]) (Table 2). On comparing the four groups of 25 consecutive patients (Table 3), a significant increase was also observed for length (5mm for group 1, 5mm for group 2, 5.6mm for group 3, and 6mm for group 4; p<0.01) and area (17.5mm2 for group 1, 15mm2 for group 2, 20mm2 for group 3, and 23.5mm2 for group 4; p<0.01).
Although fewer pneumothorax events were described for the second group of 50 patients, the difference was not significant with respect to the first group (12 [24%] vs 6 [12%], p=0.12) (Table 2). A progressive decrease in the rate of pneumothorax events was observed for the four consecutive groups of 25 patients (24% for group 1, 24% for group 2, 20% for group 3, and 4% for group 4), although the difference was not significant (p=0.06) (Table 3). No association was observed between occurrence of pneumothorax and sample length or area.
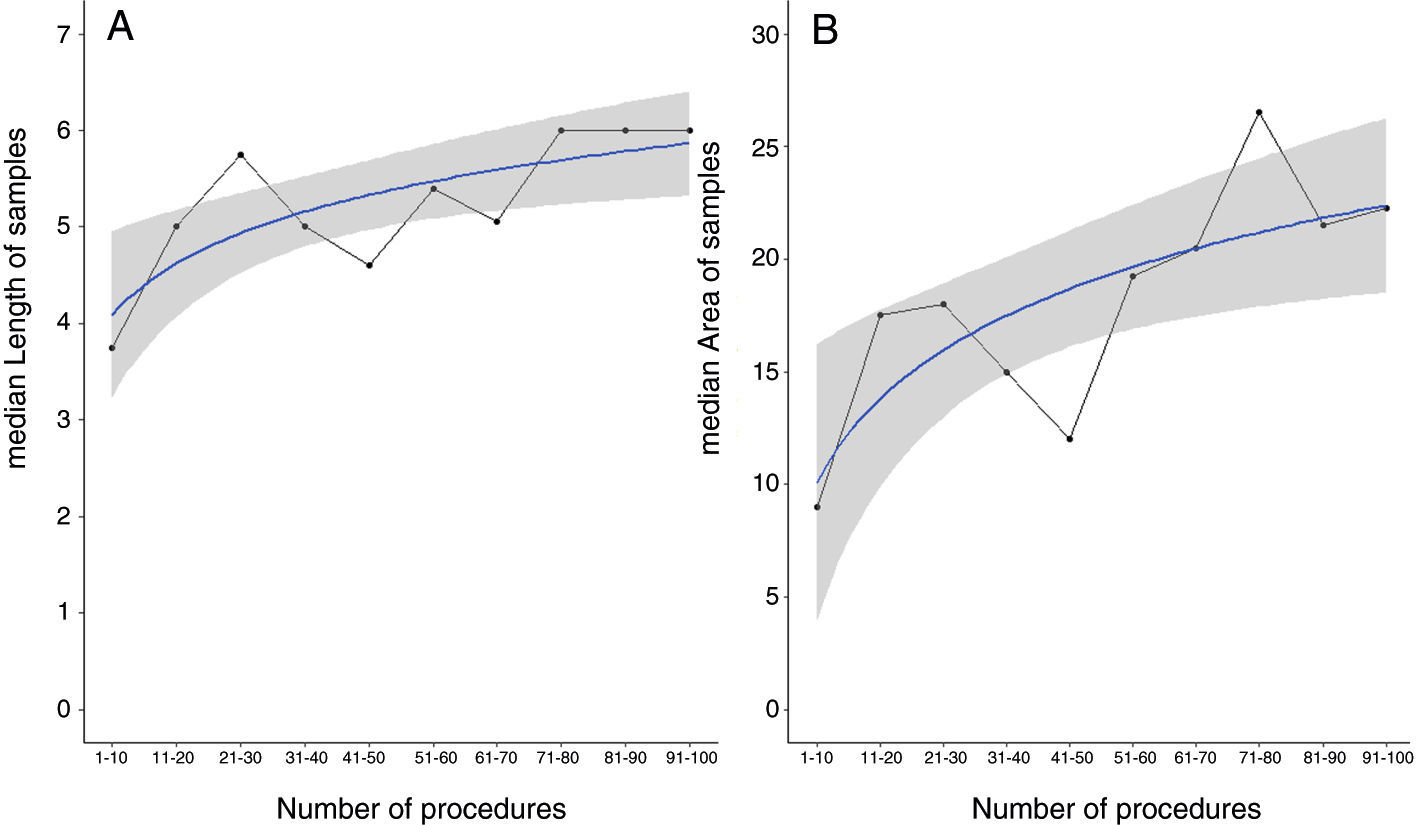
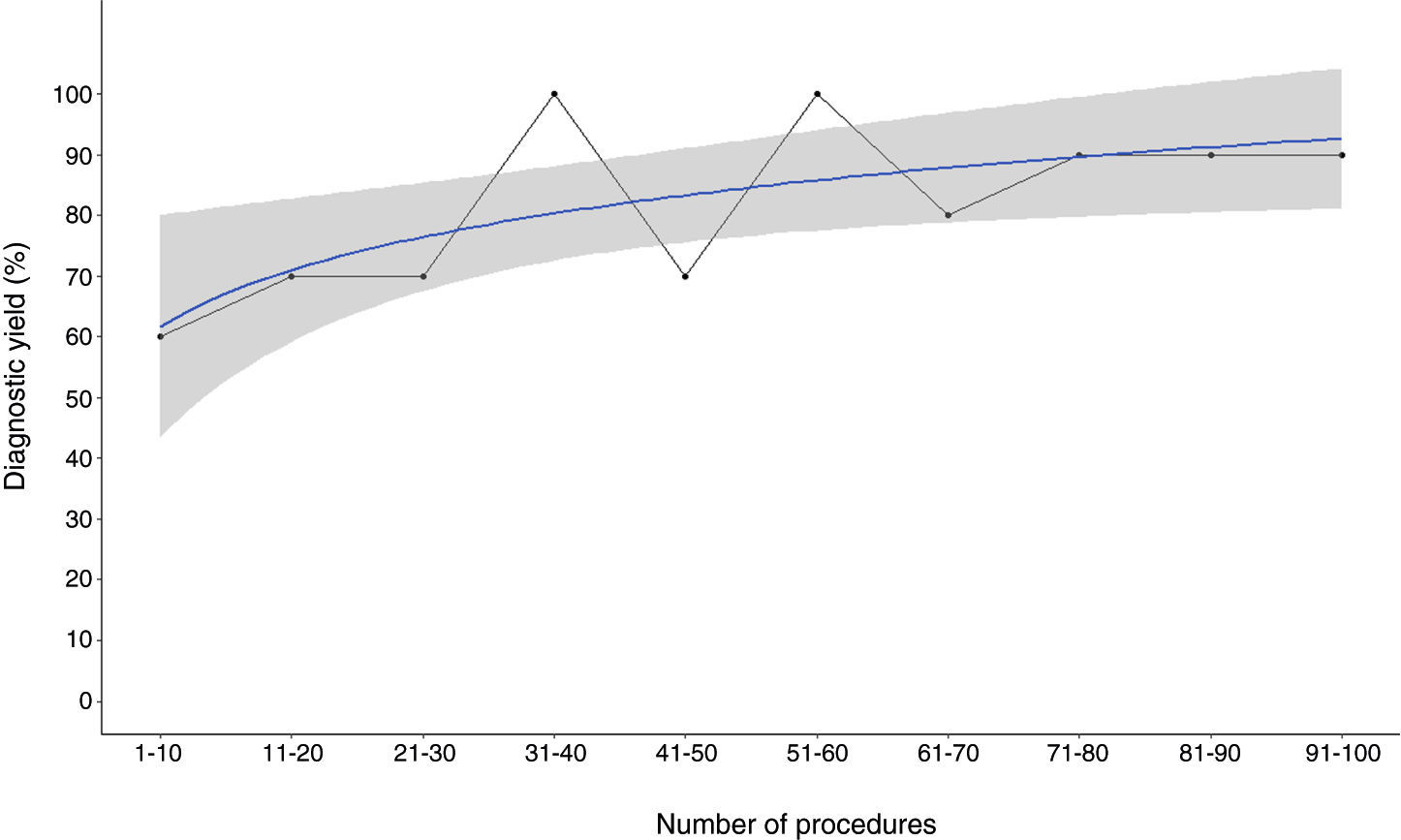
To plot the learning curve for sample area, we fitted logarithmic regressions to the median area values for each of the consecutive groups of 10 patients (Fig. 3B) and observed that the highest values corresponded to the last four groups. In the case of sample length, the learning curve plateaued after the first 70 patients (Fig. 3A). A similar threshold of approximately 70 procedures was observed for diagnostic yield (Fig. 4).
We have confirmed the high diagnostic yield of TBLC in a cohort of 100 patients from whom biopsy samples were obtained from different segments of the same lobe, as the procedure enabled a diagnosis in 82% of cases. Histologic measurements of biopsy samples showed a median length of 5.4mm and a median area of 19.5mm2. Pneumothorax occurred in 18% of patients and was clearly the main complication. On dividing the 100 patients into two groups of 50 consecutive patients, we observed a significant improvement in diagnostic yield and in sample length and area in the second group. There were also fewer pneumothoraces in the latter group, although the difference was not significant. When the cohort was divided into four subgroups of 25 consecutive patients, significant improvements in TBLC performance were noted in the last group (patients 75–100), evidenced by clear improvements in diagnostic yield (92%), median sample length (6.0mm), median area (23.5mm2), and a lower rate of pneumothoraces (4%). Based on our findings, it would appear that approximately 70 procedures are needed to achieve proficiency in TBLC.
The clinical utility of TBLC in the diagnosis of DLD has been reported by several groups, most of which reported high diagnostic performance, even in the most challenging cases (e.g., presence of a UIP pattern).8–10,13–16 Although DLD guidelines have not yet included TBLC in their diagnostic recommendations, the results clearly confirm that this endoscopic procedure is a very useful tool in MDD approaches, as it eliminates the need for more invasive procedures when histological examination is required. SLB, for example, is an invasive procedure, with attendant risks (e.g., acute exacerbation in IPF), and in addition, it is not feasible in patients with severe disease or serious comorbidities.7,22,23 In contrast the situation for other, more established, bronchoscopic diagnostic or therapeutic techniques, is that information is lacking about how much experience a bronchoscopist or hospital needs in order to properly perform, or even validate, TBLC.
Our study has some limitations. Our results are based on the analysis of data for a single hospital and a single bronchoscopist and there is therefore no information on interindividual variability. Accordingly, they cannot be easily extrapolated to other settings. Moreover, we do not use standard or universal methods (e.g., we use general anesthesia, intubation with a rigid bronchoscope, and jet ventilation), hence our results will not be readily comparable to those reported by groups who use a different approach. Nonetheless, we believe that it is important to report on individual experiences as subsequent analyses of data from different sources will help to determine the approximate threshold of cases for achieving proficiency in TBLC. Another potential limitation of our study is the fact that some patients underwent TBLC in two lobes during the study period and this could potentially have influenced the learning curve. In fact, after the first 50 patients, we modified our protocol to perform biopsies in two lobes in patients with a UIP pattern seen by HRCT. We did this because of the considerable number of patients with chronic hypersensitivity pneumonitis with UIP-like features in northern Portugal.24,25 In such cases, if samples are only taken from the usually more fibrotic lower lobe, histological examination would only reveal UIP features, failing to show the typical findings of hypersensitivity pneumonitis, which is easier to see in better preserved areas. After a rigorous evaluation, we decided not to include these cases in the current cohort, since they are characterized by substantial differences: patients usually have more serious functional decline and HRCT higher fibrotic scores; more biopsy specimens are taken when two lobes are targeted; and when a UIP pattern is present, it is important to take biopsies near to the periphery compared to other DLD. Finally, all these factors are associated with a higher risk of complications, such as pneumothorax.10,26 Another potential confounder in our study is the fact that the learning curve is dependent not only on the bronchoscopist but also on the pathologist. When TBLC is introduced into a hospital, pathologists also need to acquire expertise in evaluating samples, as they have distinctive features to those seen in SLB.27,28 The learning process therefore necessarily involves direct cooperation between the bronchoscopist and the pathologist, influencing each other.
Despite methodological differences, our diagnostic yield (82%) is in line with previous reports9,10,13,14. A recent meta-analysis of seven studies reported a diagnostic yield of between 74% and 98%, with a pooled estimate of 83% (95% CI, 73–94), when TBLC findings were interpreted in isolation. When TBLC was performed as part of a MDD diagnostic approach (8 studies), the diagnostic yield ranged between 51% and 98%, with a pooled estimate of 79% (95% CI, 65–93).14 Samples measuring at least 5mm are considered to be representative.27 Median length and area in our series are also consistent with previous reports (5.4mm and 19.5mm2 vs 3.1–8.7mm and 9.5–64.2mm2, respectively).15 These measurements in our 100 patients, however, will have been somewhat influenced by the shorter freezing time used in patients with suspected sarcoidosis (n=17), in which BAL and EBUS or/and bronchial biopsies produced inconclusive of diagnosis. The median area of samples obtained from these patients was 14.5mm2, compared with 20.2mm2 for the other patients. We opted for this shorter cooling time as diagnostic yields of between 40% and 90% have been reported in patients with sarcoidosis who underwent conventional transbronchial biopsy, where samples are usually smaller (1–3mm) and are also subject to crush artifacts.29,30 It would therefore appear that significantly larger biopsy samples are not required in such cases in order to establish a diagnosis. This theory appears to be sustained by our data, since all patients with suspected sarcoidosis had histologic features that provided a definite diagnosis by TBLC. Furthermore, there were two cases of pneumothorax which correspond to 11.7% of the cases with final diagnosis of sarcoidosis which is similar to what is usually reported in conventional transbronchial biopsies. Additionally, any case with significant bleeding in the patients had been recorded. We can speculate that the most cautious approach that we usually take in cases with sarcoidosis suspicion could help to avoid those complications.
Our series can also be considered representative of DLD, as we analyzed a heterogeneous range of some of the most common disorders, including chronic hypersensitivity pneumonitis, sarcoidosis, interstitial pneumonias as UIP, NSIP, smoking-related pneumonias, and organizing pneumonia. Analysis of our data also showed that these disorders were randomly distributed over the period analyzed.
Pneumothorax was the most common complication in our series. Reported incidence rates vary considerably (0% to approximately 30%),14,15 possibly due to differences in procedure (e.g., distance from the pleura) or in types of disorders (e.g., some series have more fibrotic pneumonias).10 The complication rate of 18% detected in our series could also be related to our particular approach, as we usually take samples from a distance of 10mm from the pleura. It might also be explained by the considerable number of cases of fibrotic pneumonia (e.g., chronic hypersensitivity pneumonitis and UIP) or smoking-related pneumonia, which is sometimes associated with emphysema, however there was no association with a particular histology pattern with pneumothorax. Bleeding is a relatively frequent event in TBLC, but is usually kept under control when a Fogarty balloon is used. We consider mild bleeding to be a standard part of TBLC and therefore did not record it as a complication. Only 3% of patients experienced moderate bleeding, which was stopped with adrenaline, cold saline, or intubation of the biopsied segment with the bronchoscope. No cases of severe hemorrhage were observed. We used the BTS classification for bleeding during bronchoscopy as this is the standard system used in our department and we feel it to be adequate. Bleeding rates for moderate/severe bleeding associated with TBLC vary considerably in the literature, with rates ranging from 0% to 78%. This wide range can probably be explained by the use of different approaches and/or bleeding classification systems.14 What does seem clear, however, is that bleeding complications are significantly reduced when a Fogarty balloon is used.10,31
Overall, our findings for diagnostic yield, sample length, sample area, and rate of complications for TBLC performed in 100 consecutive patients suggest that approximately 70 procedures are needed for a bronchoscopist to acquire the desired level of expertise. This figure is similar to numbers reported for other endoscopic diagnostic techniques, such as conventional transbronchial needle aspiration and even endobronchial ultrasonography, for which proficiency is attained after a threshold of approximately 50 and 70 procedures respectively.18,20
In conclusion, despite the limitations of the current study, in particular our focus on a single bronchoscopist and hospital, we suggest that the learning curve for TBLC plateau after approximately 70 procedures. More studies are necessary to determine this threshold and guide bronchoscopists starting out with this endoscopic diagnostic technique.
Author contributionsL.M. Almeida: Substantial contribution to conception and design, analysis and interpretation of data, drafting the article, and finalizing the version to be published.
B. Lima: Statistic analysis and substantial contribution to interpretation of data, drafting statistic and results section.
P.C. Mota: Contribution to analysis and interpretation of data.
N. Melo: Contribution to analysis and interpretation of data.
A. Magalhães: Contribution to analysis and interpretation of data.
J.M. Pereira: Contribution to analysis and interpretation of data.
C.S. Moura: Contribution to analysis and interpretation of data.
S. Guimarães: Contribution to analysis and interpretation of data.
A. Morais: Substantial contribution to conception and design, analysis and interpretation of data, drafting the article, and finalizing the version to be published.
Conflicts of interestNone declared.