The effectiveness and safety of macitentan, a dual endothelin-receptor antagonist (ERA) approved for the treatment of pulmonary arterial hypertension (PAH), were shown in an extensive clinical trial oriented towards morbidity and mortality events. Our aim was to describe a single centre's experience of the utilization of macitentan in patients with PAH in clinical practice settings. Thirteen patients with different aetiologies and previous PAH treatments were studied. After 12 months of macitentan treatment, 11 patients improved their functional class (FC), all patients improved their 6-minute walk distance (6MWD) test, and 10 patients lowered their NT-proBNP plasma levels. Additionally, cardiac imaging parameters were also improved. No cases resulted in hospitalization, septostomy, transplant or death.
Macitentan is an ERA showing enhanced tissue penetration, higher pKa,1 increased ET-receptor occupancy2 and superior oral efficacy in animal models compared to other ERAs.1 It also shows minimal risk of relevant drug-drug interactions and low risk of hepatotoxicity when administered for PAH.3–5 Macitentan is the first drug for PAH approved after a long-term, event-oriented clinical trial, showing a decrease in morbidity and mortality.4 Macitentan is recommended as monotherapy or in sequential combination for PAH patients in WHO-FC II and III in the latest European ESC/ERS guidelines for pulmonary hypertension (PH).6 These guidelines established a new assessment of 1-year mortality risk in PAH patients, classifying patients as low, intermediate or high risk, taking into account clinical parameters, exercise, cardiac imaging and haemodynamics.6 However, other parameters such as age, sex or PH aetiology are not considered. The validity of most comprised parameters has been demonstrated in risk assessment at diagnosis but their validity during patients’ follow-up is uncertain. Our aim was to describe a single centre's experience of the 1-year utilization of macitentan in patients with PAH in the clinical practice setting.
MethodsRetrospective series of PAH patients in WHO-FC II or III treated with macitentan under routine clinical practice in the Pulmonary Hypertension Unit of the Complejo Asistencial Universitario de Salamanca (Spain). This is a multidisciplinary unit coordinated by the pulmonology and cardiology Services, with 4 specialized outpatient visits each week, including 1 congenital visit; and over 100 patients attended.
We recorded available clinical, cardiac imaging and haemodynamic variables at baseline and after 12 months of treatment with macitentan, and drug safety. We categorized the registered clinical variables at baseline and 12-months in low-, medium- and high-risk categories using the risk assessment table published in the 2015 ESC/ERS guidelines.6 This classification serves as a mere reference given the complexity of PAH and the limited applicability of proposed cut-off values at a case-by-case level in real-world practice. This study was approved by the Hospital Ethics Committee. All participants provided written informed consent.
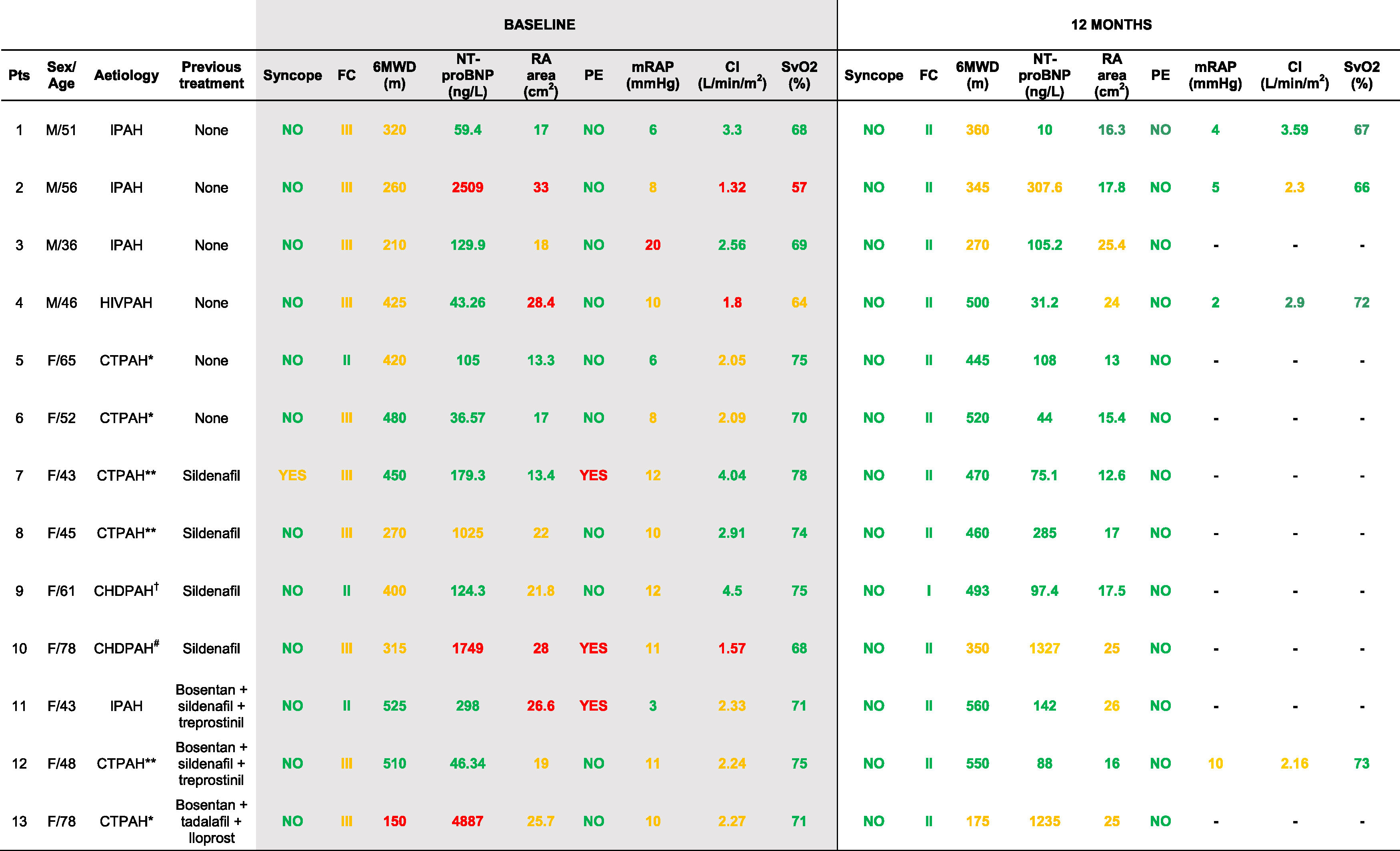
ResultsThirteen patients, (6 naïve), were treated with macitentan for 12 months between September/2014 and October/2016. The demographic and clinical characteristics, and administered treatments at baseline and after 12 months are shown in Table 1. Six patients were treatment-naïve and 4 started sequential combination with macitentan plus sildenafil after not reaching therapeutic objectives with sildenafil alone. Additionally, three patients who received triple-combination therapy, including systemic prostaglandins (N=2) and inhaled iloprost (N=1), started macitentan due to hepatotoxicity of other ERAs. These patients were included in this analysis as they remained on stable doses of prostaglandins and diuretics during the treatment period. Patients with connective tissue associated PAH presented limited form of systemic scleroderma (Patients 5, 6 and 13) or systemic lupus erythematosus (SLE) (Patients 7, 8 and 12). Patient 9 presented ventricular septal defect and Patient 10 atrial septal defect, with persistent PAH after defect correction. One patient (Patient 4) presented HIV infection associated PAH and four patients (Patients 1, 2, 3, and 11) were diagnosed with idiopathic PAH.
Patient characteristics, PAH aetiologies and clinical variables at baseline and after 12 months of treatment with macitentan.
Risk category is indicated according to ESC/ERS 2015 guidelines criteria as follows: Green (low risk), yellow (intermediate risk), red (high risk). FC (New York Heart Association functional class); 6MWD (6-minute walk distance test); NT-proBNP: N-terminal pro-brain natriuretic peptide; RA (right atrial); PE, pericardial effusion; mRAP (mean right atrial pressure); CI (cardiac index); SvO2 (mixed venous oxygen saturation); IPAH (idiopathic pulmonary arterial hypertension); CTPAH (connective tissue associated PAH); HIVPAH (HIV infection associated PAH); CHDPAH (congenital heart disease associated PAH).
*Associated to a limited form of systemic scleroderma.
**Associated to systemic lupus erythematosus.
†Repaired interventricular communication with associated pulmonary hypertension.
#Repaired interatrial communication with remaining pulmonary hypertension; –, not done.
Among 13 included patients, 11 improved their FC, 13 their 6MWD (average: 55m), and 10 lowered their NT-proBNP plasma levels (average: −31%). Regarding cardiac imaging, 11 patients reduced their right atrial area, whereas the pericardial effusion observed in three patients at baseline was not present after 12 months (Table 1). Haemodinamic parameters were not available in all patients at 12 months given their favourable disease progression.
After 12 months of treatment with macitentan no cases resulted in hospitalization, septostomy, transplant or death. Two patients required additional therapies for PAH: After an initial clinical improvement, Patient 2 required a rapid step-up to triple therapy at month 6 due to clinical worsening (imaging/haemodynamic confirmation), achieving positive outcome. Patient 4 added a phosphodiesterase-5 inhibitor after failing to meet therapeutic objectives (imaging parameters, month 5).
Safety profileTwo patients reported cephalea. Patient 8 had 10g/dL of baseline haemoglobin and developed anaemia during the study due to: SLE, hypersplenism and a potential effect of the drug. There were no treatment discontinuations or hospitalizations caused by PAH worsening. In patient 4 (HIV-associated PAH), viral load and antiretroviral (tenofovir, darunavir, ritonavir, raltegravir) and CD4 levels were closely monitored without detecting interactions with the baseline treatment. There were no oedema or transaminase elevation events.
DiscussionOur results from a single-centre study showed a noticeable improvement in clinical observations after 12 months of macitentan treatment in patients with PAH in clinical practice settings. This favourable evolution was observed across aetiologies and therapeutic strategies, and irrespective of previous treatments. Increasingly, the clinical improvements were also observed in the 3 patients previously treated with bosentan, and were associated with a good tolerability, aligned with recently published reports,7–9 without transaminase elevation events.
After 12 months of treatment, no cases resulted in hospitalization due to PAH, septostomy, transplant or death. These results are consistent with the composite secondary endpoint (reduction death due to PAH or hospitalization for PAH) of the SERAPHIN trial, in which the risk of such events was significantly reduced by 50% (p<0.001),4 although comparisons have to be made with caution given the disparity of the population size and characteristics.
Current treatment for PAH is not a mere long-term administration of drugs. Guidelines recommend a close monitoring and an individualized management of patients.6 The treatment goal in patients with PAH is achieving a low risk status, associated to a 1-year mortality risk <5%.6 However, this risk classification and the therapeutic objectives are not yet manifest and have to be defined in subsequent studies.10 They are also increasingly ambitious, not always realistic for certain patients, which bolsters the relevance of our results. Although patients were treated on a clinical practice basis and had different disease histories and baseline characteristics, after 1 year of macitentan treatment none of the measured parameters were classified under the high risk category as defined per 2015 ESC/ERS guidelines. Furthermore, all parameters classified as intermediate risk maintained or improved their risk category, and the low risk variables remained under such classification.
Also of worth noting is the good safety and tolerability observed. The stability in the viral load and CD4 levels and the absence of drug-drug interactions between macitentan and the antiretrovirals used in Patient 4 indicates a safety advantage in this highly medicated patient profile. Neither macitentan nor its active metabolite has clinically relevant inductor or inhibitory effects on the P450 cytochrome enzymes, and macitentan has a favourable hepatic safety profile compared to other ERAs.11
Study limitations include: the reduced number of patients, single-centre experience, and retrospective analysis, limiting its significance and potency. Also, follow-up catheterisation was not available for all cases as the majority progressed favourably and there is a lack of evidence that a regular catheterisation in treated patients is associated with increased survival, especially if the other parameters are at low to intermediate risk levels.6 In our clinical practice, catheterisation is not systematically performed unless we observed an unexplained clinical worsening or a treatment decision can be expected from the results. However, we perform periodical catheterizations in the most severe cases, framed in their close monitoring programmes and regardless of other parameters’ results. It is important to note that in those patients who did not receive catheterisation at 12 months, their baseline poor prognosis was not solely determined by haemodynamic criteria, with the exception of Patient 3, who had no vascular access at 12 months and presented Prader-Willi syndrome.
In conclusion, we observed an improvement in the achievement of therapeutic objectives in a series of patients with different PAH subtypes treated for 12 months with macitentan, with a very good safety profile. These improvements were observed irrespective of patient's therapeutic strategies and previous treatments. Further controlled studies with extended follow-up periods are required to corroborate these results.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestS. Cadenas-Menéndez has received research funding and acted in consultancy/speaker roles for Actelion. M.A. Gomez-Sanchez has received honorariums for consultations and/or speaking at conferences from Actelion, Bayer, GSK, MSD and Pfizer. The rest of authors declare no conflict of interest.
Medical writing assistance: Juan Martín Alonso (TFS), funded by Actelion Spain.
Actelion did not have any role in the collection, analysis and interpretation of data; writing the report; and the decision to submit the report for publication. The views expressed are therefore based on authors’ opinions and do not represent the views of Actelion or the Journal.