Allergic rhinitis (AR) and asthma are two common chronic diseases that often coexist. There is a need for a validated tool to evaluate HRQoL of Portuguese speakers with asthma and/or rhinitis patients in clinical practice.
ObjectivesTo adapt and validate RhinAsthma Patient Perspective (RAPP) in Portuguese.
MethodsThe RAPP questionnaire was translated into Portuguese. Asthmatics with comorbidities and rhinitis attending the allergy department of Coimbra University Hospital were asked to complete the Portuguese translation of RAPP, in addition to the SF-12, ACT, and a Symptomatologic VAS twice, with a 4-week interval between visits. During Visit 2, a Global Rating Scale (GRS) was completed to assess any change in health status. Scale dimensions, internal consistency and convergent validity, reliability, discriminant ability and responsiveness to change, as well as Minimal Clinical Difference were assessed.
ResultsFactor and confirmatory analysis confirm the unidimensional structure of the questionnaire. Internal consistency has been shown to be satisfactory (0.82 visit 1 and 0.86 at visit 2). The tool is able to discriminate between patients on the basis of asthma severity, asthma control level, and rhinitis severity; convergent validity showed a significant correlation with SF-2 Physical component (r=−0.46 and 0.42, p at Visits 1 and 2). An ICC of 0.97 and a CCC=0.94 indicate that the tool is highly reliable. Responsiveness was shown in detecting a significant association with GRS changes (r=0.41, p<0.01) and ACT (r=−0.47, p<0.01) but not with VAS. (r=.14, n.s.). MID value was 2 points.
ConclusionsThe Portuguese version of RAPP has been demonstrated to have good measurement properties and sensitivity to health changes, which will provide a valid, reliable and standardized HRQoL measurement in patients with asthma and comorbid allergic rhinitis in clinical practice.
Allergic rhinitis (AR) and asthma are two common chronic diseases that often coexist: up to 80% of patients with asthma suffer from AR, while 10–40% of patients with AR also have asthma.1–4 These diseases represent a significant socio-economic burden on both individuals and society as a whole due to the high direct and indirect costs.5 Moreover, there is now considerable evidence that AR and asthma significantly impair Health Related Quality of Life (HRQoL). In fact, the availability of validated questionnaires has assisted the measurement of the impact of respiratory allergies from a patient perspective and has produced a rich literature on the ways that asthma and AR negatively affect patient physical, emotional, mental and social life.5,6 However, if HRQoL has become increasingly considered as an outcome measure in clinical trials, its integration into routine assessment remains challenging. This depends primarily on the lack of questionnaires that are specifically validated for use with individual patients.
RhinAsthma Patient Perspective (RAPP) is a validated tool of 8 questions, available in Italian,7,8 that provides evaluation of HRQoL of patients with asthma and/or rhinitis in clinical practice. Patients are asked to grade the extent to which they have been bothered by each problem during the previous 2 weeks using a 5-point Likert-type scale (not at all, a little, quite, a lot, and very much). The tool is simple to complete and the score is a simple sum of the single answers (range 8–40). A cutoff point of 15 demonstrated the best sensitivity and specificity in discriminating the achievement of an optimal HRQoL. The RAPP owns the psychometric properties that are requested for the use of an instrument with an individual patient.9,10
The aim of this study was to cross-culturally adapt and validate the RAPP in Portuguese. This project was part of a larger multinational study aimed at evaluating the psychometric properties of the RAPP in five countries (Spain, France, Portugal, Poland and the Philippines) and to compare HRQoL in the countries involved.
Materials and methodsThe process of cross-cultural adaptation was conducted according to international guidelines with 2 forward and backward translations.11 Once the Portuguese version was obtained, patients who visited the Immunoallergology Department, Coimbra University Hospital, Portugal, were prospectively enrolled between June and December 2017. Data was collected using a convenience sampling method.12 The aim was to include 150 patients. The inclusion criteria were: age ≥18, asthma and/or rhinitis diagnosis according to GINA and ARIA guideline and willingness to be enrolled into the study. Patients suffering from any other respiratory or ear–nose–throat disorders were excluded.
The study was approved by the ethics committee of the University of Genoa (approval no. P.R. 333REG2016) and ratified by the local ethics committee; it was conducted in accordance with the general principles of Good Clinical Practice and the Declaration of Helsinki as amended in Edinburgh in 2000. Each patient gave written informed consent to participate at the beginning of the study.
Patients were assessed twice, with a 4-week interval between visits. At the first visit, a physician collected a complete and accurate medical history and recorded ongoing therapy. Their last available spirometric value was registered. Smoking status was collected and patients were classified as current smokers, former smokers or non-smokers. At each visit patients filled in the RAPP questionnaire, SF-12 to assess health status, asthma control test (ACT), and a Symptomatologic Visual Analog Scale (VAS) to evaluate rhinitis severity. At Visit 2, a Global Rating Scale was completed to evaluate any change in health status.
In order to validate the Portuguese version of RAPP, the following psychometric analyses were performed:
- 1)
Scale's dimension by mean of explorative and confirmative factor analysis.13,14 In more detail, the Kaiser–Meyer–Olkin (KMO) test was adopted to analyze the feasibility of factor analysis, and Bartlett's Test of Sphericity was chosen to test for null hypothesis that the correlation matrix has an identity matrix. The root-mean-square error of approximation (RMSEA), comparative fit index (CFI), and standardized root mean squared residual (SRMR) were used to assess fit.
- 2)
Internal consistency was measured using Cronbach's alpha on the whole test. Values >0.70 are generally considered acceptable,15 whereas higher scores are recommended for use in an individual patient.16
- 3)
Reliability was assessed in patients with a stable health status (GRS=0) by means of interclass coefficient (ICC) and Lin's concordance correlation coefficient (CCC).17 Coefficients of 0.70 for group comparisons and of 0.90 for comparisons within individuals are recommended.13
- 4)
Convergent validity was evaluated using Spearman's between RAPP and SF-12. Correlations ranged from 0.4 to 0.8 confirm the convergent validity.
- 5)
Discriminant validity was assessed by means of ANOVA (Fischer's test) comparing patients according to ACT, GINA and ARIA classification of severity.
- 6)
Responsiveness was evaluated by analyzing the correlation between changes in RAPP scores and changes in GRS, VAS and ACT by means of a non-parametric test (Spearman correlation coefficient).
- 7)
Minimal important difference (MID) was determined by applying the receiver operating characteristics (ROC) curve method. The entire cohort for one dichotomization point (i.e., ‘no change’ vs ‘any improvement or deterioration’) was adopted.18
The possible effect of age, education, and smoking habits on patients’ answers was explored by means of Spearman's correlation coefficient and ANOVA Fischer's test.
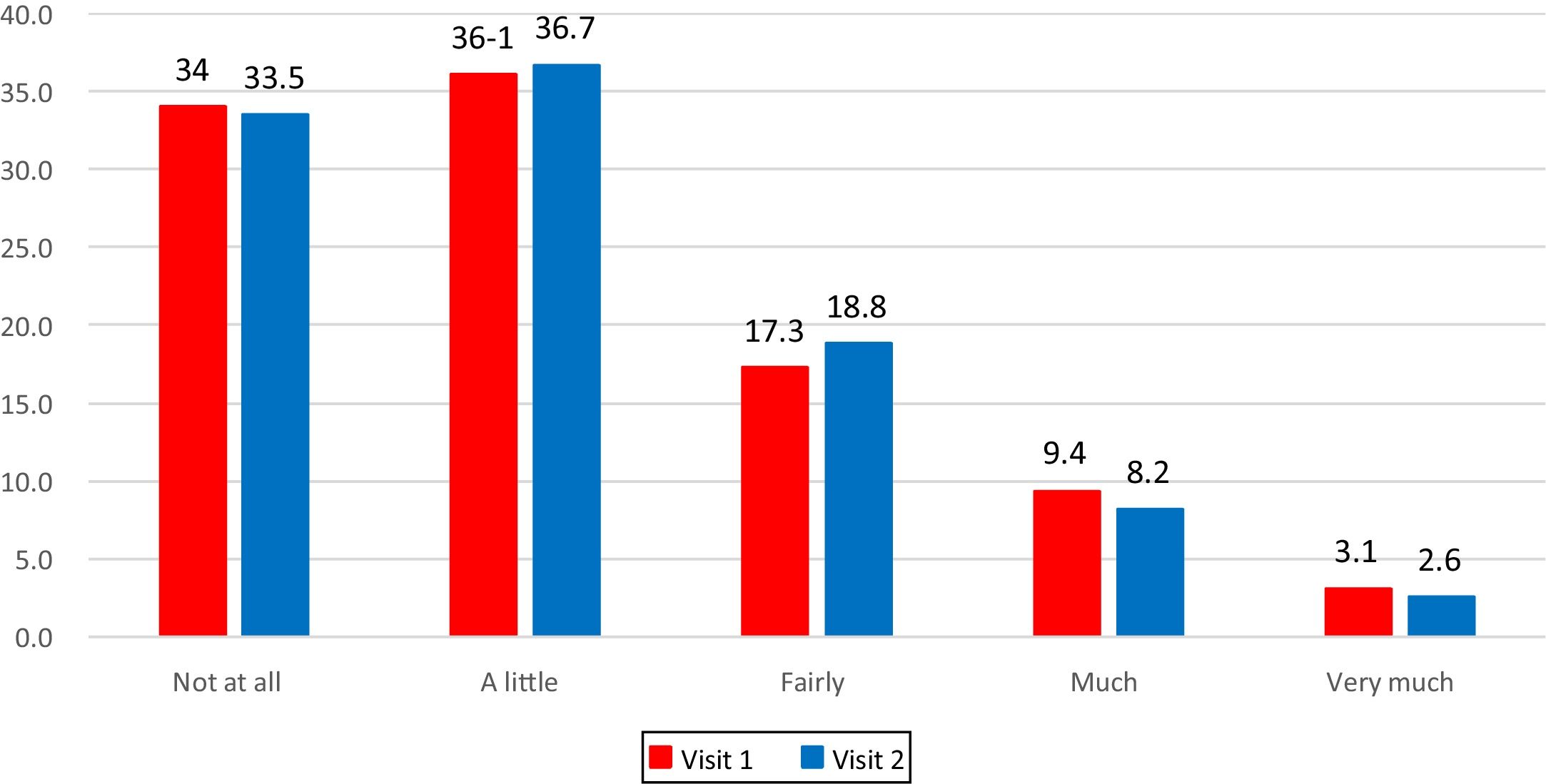
The frequency distribution of answers was calculated to verify whether patients were using the entire answer scale.
Statistical analysesStatistical analyses were performed using IBM SPSS Statistics, version 24, Armonk, NY while confirmatory factor analysis was performed using Mplus 7.0 (Muthén & Muthén, Los Angeles, CA).19
ResultsThe validation sample consisted of 149 patients (65% females). The mean age was 36.8 (age range 18–81 years). Of the respondents, 4.2% were current smokers, 10.3% former smokers and 85.5% non-smokers. Academic degree was the most common level of education attained (55.3%), followed by high school (36.6%), secondary school (6.5%) and primary school (1.6%) qualifications. 61.1% of patients suffered from persistent asthma and 38.9% from intermittent asthma. AR was classified as mild intermittent in 24.2% of all cases, moderate–severe intermittent in 3.6%, mild persistent in 14.8%, and moderate–severe persistent in 56.4% according to ARIA guidelines.1 The ACT score at Visit 1 showed 31.7% to be totally controlled, 49.7% to be well controlled, and 18.6% to be uncontrolled. The effective time between the two visits was 28±3 days.
The mean value of AR and asthma quality of life was 16.9±5.5 at visit 1 and 16.8±5.6 at visit 2.
- 1)
Scale dimensions confirmed that the data were suitable for factor analysis. The solution revealed a unidimensional structure that absorbed 37.8% of the total variance and only 4 residuals greater than |0.10| at visit 1 and 43.4% of the total variance and only 2 residuals greater than |0.10| at visit 2. The unidimensional structure was confirmed: the goodness-of-fit indexes were all satisfactory both at visit 1 (RMSEA 0.08, SRMR 0.05, CFI 0.91) and at visit 2 (RMSEA 0.09, SRMR 0.06, CFI 0.90)
- 2)
Internal consistency: Cronbach alpha values were 0.82 at visit 1 and 0.86 at visit 2, both satisfactory.
- 3)
Reliability was evaluated in 43 patients reporting an unchanged health status (GRS=0) showing an ICC of 0.97 and a CCC=0.94.
- 4)
Convergent validity: correlations between RAPP scores and the Physical Component Score of SF-12 were significant both at Visit 1 (r=−0.42, p<0.01) and at Visit 2 (r=−0.46, p<0.01), while correlations were not significant between RAPP and the Mental Component Score of SF-12 either at Visit 1 (r=−0.05, n.s.) or Visit 2 (r=−0.02, n.s.).
- 5)
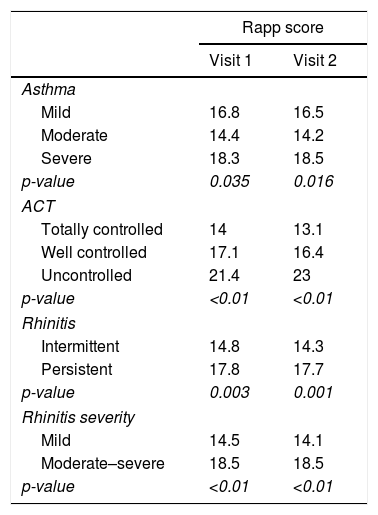
Discriminant validity: RAPP was able to discriminate between patients on the basis of asthma severity, asthma control level, and rhinitis severity (Table 1).
Table 1.(RAPP) discriminant validity.
Rapp score Visit 1 Visit 2 Asthma Mild 16.8 16.5 Moderate 14.4 14.2 Severe 18.3 18.5 p-value 0.035 0.016 ACT Totally controlled 14 13.1 Well controlled 17.1 16.4 Uncontrolled 21.4 23 p-value <0.01 <0.01 Rhinitis Intermittent 14.8 14.3 Persistent 17.8 17.7 p-value 0.003 0.001 Rhinitis severity Mild 14.5 14.1 Moderate–severe 18.5 18.5 p-value <0.01 <0.01 - 6)
Responsiveness was assessed in 106 patients with a health improvement or deterioration, evaluated by GRS≠0. RAPP was significantly associated with changes in GRS (r=0.41, p<0.01) and ACT (r=−0.47, p<0.01) while the association with VAS was not significant (r=.14, n.s.).
- 7)
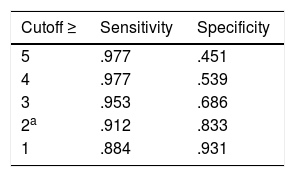
MID: A 2 point difference or change in RAPP maximizes sensitivity, specificity, and the number of individuals correctly classified (Table 2).
Table 2.The MID of RAPP obtained with the ROC analysis with different cutoff.
Cutoff ≥ Sensitivity Specificity 5 .977 .451 4 .977 .539 3 .953 .686 2a .912 .833 1 .884 .931
No significant difference was reported in RAPP scores between smokers, former smokers, and nonsmokers (ANOVA Fisher's test. Visit 1: p=0.06; Visit 2: p=0.11) nor on the basis of level of education (ANOVA Fisher's test. Visit1: p=0.679; Visit 2: p=0.476). Significant associations were found between age and RAPP scores (Spearman's correlation. Visit 1: r=0.23, p=0.005; Visit 2, r=0.31, p<0.01).
The frequency distribution of answers at Visit 1 and at Visit 2 shows that the entire scale range had been used (Fig. 1).
DiscussionIn daily clinical practice, therapeutic management of patients with comorbid asthma and allergic rhinitis is primarily decided by the physician according to the patient's symptoms and biological parameters. Little emphasis is given to the patients’ perspective of the impact of their disease and its treatment upon their quality of life. This is mainly due to the lack of simple and reliable tools for assessing HRQoL in daily routine; the questionnaires available are too long and complex for routine clinical use.20
RAPP has been validated in Italian according to the available guidance to properly assess the patient's perspective in clinical practice21,22 and a cross-cultural validation is needed to use this tool in everyday practice in other languages. To perform this process we selected specific tools that are widely used for the validation process of HRQoL questionnaires and which are available in the languages needed for all countries involved in this international study. For this reason, it was not possible to assess the level of control both in asthma and AR. In fact, although tools specifically designed to assess control are available for asthma (ACT), rhinitis (ARCT)23 and concomitant asthma and rhinitis (e.g. CARAT),24 only for the ACT do we have a validated version in Spain, France, Portugal, Poland and the Philippines. For this reason, we decided to include the ACT in our protocol and to evaluate symptom severity instead of control in AR, by mean of VAS. This prospective study was performed to validate the Portuguese version.
We found the Portuguese version of the RAPP to be reliable for the individual assessment of HRQL. Our analyses confirmed the unidimensional structure of the tool and the results of factorial analysis explain more than 40% of variance, proving its strong validity.
In terms of reliability, the Portuguese version of the RAPP performed well in the present validation study, with Cronbach-α values over the recommended 0.70 threshold for the overall score. Internal consistency results were generally comparable with, or better than, those seen for the original instrument. Satisfactory convergent and discriminant validity and high responsiveness to changes were confirmed. The lack of effect of demographic characteristics on patients’ answers makes the questionnaire appropriate for use in everyday practice.
Since asthma and rhinitis symptoms can vary over time, the availability of a patient-reported outcomes measure which is capable of mirroring these changes is significant. As in the original version, a 2 point change in RAPP score identified a HRQL change perceived by the patient.
The present study has several strengths. First of all it confirms the psychometric properties of RAPP in a language different from the original one. Moreover it offers the possibility of assessing HRQoL in the routine clinical management of Portuguese patients with asthma and AR and to compare the HRQoL of the Portuguese-speaking population with other patients from different countries.
One limitation of our study is the generalizability of the results: patient selection bias cannot be excluded since the patients were recruited from one single specialist center and the sample was nonrandomized. Moreover, the acceptability of the RAPP for both patients and physicians and the added value of using this tool in clinical practice have not been assessed. These limitations may be addressed through further studies including other settings and by assessing acceptability and clinical relevance.
In conclusion, this study demonstrates that the Portuguese RAPP version is suitable for use among asthma and allergic rhinitis patients both in research and clinical settings.
FundingThis work was supported by Menarini International Operations Luxemburg S.A. (M.I.O.L.). Contract ARMIA_M.I.O.L. 19/09/2016.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank ARMIA (Associazione Ricerca Malattie Immunologiche e Allergiche) for scientific support.