Dear editor,
The broad antibacterial activities of macrolides have led to their widespread use on infections, particularly for respiratory infections. Many of the macrolide-susceptible microorganisms are respiratory pathogens known to be associated with exacerbations of asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis (BE) and cystic fibrosis (CF). Macrolides have been widely used to treat respiratory diseases, often beyond its acute antimicrobial use (Table 1), and they have long been accepted to show effects on inflammatory and immune cells, mucus secretion, and epithelial cell differentiation. Anti-inflammatory and immuno-modulatory actions, independent of their actions on bacteria, add to their therapeutic benefit in infectious and chronic inflammatory disorders, recognition that dates to the 1960s.1 Long-term treatment improves clinical outcomes in several respiratory diseases, such as in symptoms, lung function, quality of life (QoL) and a significant exacerbation frequency reduction in COPD (30 to 60 %), asthma, CF and BE, despite diversity in pathophysiology between these conditions, suggesting a degree of shared biology.2 Thus, as macrolides show a positive effect in diseases where macrolide-resistant organisms are prevailing, and at dosages below the antibacterial threshold, this suggests that this effect may not be a result of antibacterial activity. This is further highlighted by reports that airway bacterial load remains unchanged.1 However, up till now, there has been limited success in defining the essential molecular mechanisms underlying these activities. By understanding them, it might be possible to enhance their non-antibiotic functions, apply them to other inflammatory diseases, avoid side effects, and limit bacterial resistance. Clinical studies exploring the long-term effects of macrolides in chronic lung diseases have reported adverse events including hearing impairment, gastrointestinal disarrangement and, although rare, cardiotoxicity – extension of QT interval (reported in several COVID-19 trials of azithromycin (AZM)) and inhibition of proarrhythmogenic drugs metabolism, leading to syncope and sudden death. The long-term use is also limited by the induction of bacterial resistance, and there is a concern about the length of low-dose macrolide therapy that starts to increase bacterial resistance. Thus, there is a need for new nonantibacterial macrolides that have similar effective disease-modifying properties without the risk of resistance.1 It is reported that macrolides reduce immune cell infiltration into the lungs of asthmatics and BE patients, and it is suggested that leukocyte migration into the lung is restricted by reducing chemokine and adhesion molecule production by airway epithelial cells.2 Multiple potential effects on macrophage function have been described: macrophage phenotype depends largely on the cytokine environment and is simplified to pro-inflammatory (M1-like) and anti-inflammatory (M2-like). Macrolides are consistently reported to direct macrophage precursors and existing M1 cells towards an M2 phenotype in vitro and subsequently change macrophage cytokine production, to enhance the phagocytic capacity and efferocytosis - the phagocytic clearance of dead cells - as apoptotic bronchial epithelial cells and neutrophils. This may be partially responsible for macrolide efficacy in COPD, where impaired clearance of apoptotic bronchial epithelial cells is considered a key part of the pathophysiology.2
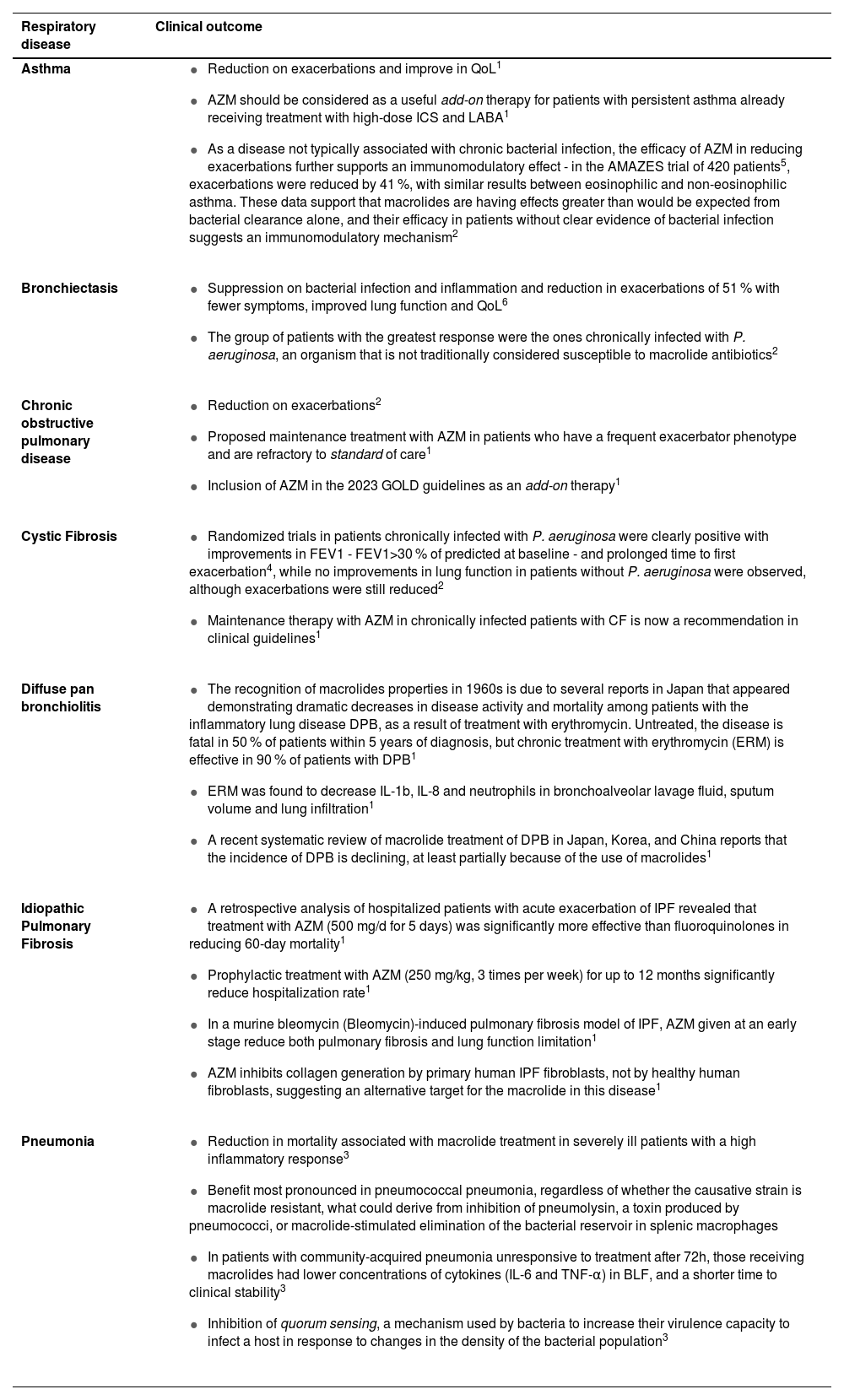
Clinical outcomes of macrolides according to each respiratory disease.
| Respiratory disease | Clinical outcome |
|---|---|
| Asthma |
|
| Bronchiectasis |
|
| Chronic obstructive pulmonary disease | |
| Cystic Fibrosis |
|
| Diffuse pan bronchiolitis |
|
| Idiopathic Pulmonary Fibrosis |
|
| Pneumonia |
|
Neutrophils eliminate infection in a variety of ways including phagocytosis, degranulation and the formation of neutrophil extracellular traps. It is thought that a central mechanism behind the positive clinical outcomes of macrolide treatment is the mitigation of neutrophil responses. Prolonged neutrophil lifespan caused by delayed apoptosis is thought to be prominent in many chronic diseases, and macrolides shorten it by inducing apoptosis, reducing the likelihood of cells undergoing necrosis and releasing inflammatory mediators into the local lung environment. Enhanced neutrophil apoptosis together with the macrolide-induced enhanced efferocytosis capacity of macrophages promotes an anti-inflammatory environment.1 Dendritic cell (DC) phenotypic plasticity allows for regulation of immune responses, often perturbed in inflammatory disease where DCs extend chronic inflammation and tissue damage. Macrolides modulate T-cell function both directly and indirectly - induction of a tolerogenic-like DC phenotype is likely to suppress T-cell activation and proliferation or induce formation of anti-inflammatory Treg- cells. Low-dose macrolide therapy might increase apoptosis of specific T-cell subsets, highlighted by decreased CD8+ and unchanged CD4+ in macrolide-treated patients, what may be a consequence of the reduction in pro-inflammatory cytokines involved in recruitment and proliferation, rather than a direct effect of macrolides on T-cell.2 Macrolides can be modified to eliminate their antibacterial effects while keeping or enhancing their immunomodulatory capacity, and can also be coupled with other molecules, such as steroids, antimicrobial peptides, or small signalling molecules to enhance their potency. Given that macrolides are relatively stable in vivo and accumulate in phagocytes, they are excellent carrier molecules to deliver drugs or signals specifically to phagosomes or inflammation places.3 Although taking advantage of the beneficial immunomodulatory effects of macrolides is an appealing perspective, clinical evidence is scarce at present, and caution is required in terms of safety and antimicrobial resistance. Further studies are needed to verify whether this could be a valid therapeutic, to establish which clinical or immunological subsets of patients might benefit the most, and to further improve in new non-antibiotic macrolides, while preserving the use of macrolides as antibiotics.3
CRediT authorship contribution statementDaniela Godinho: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Marta Freixa: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Filipe Froes: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.