If the seemingly less invasive semi-flexible pleuroscopes are combined with strategies of conscious sedation and local anesthesia the pleuroscopy has the potential to reach an increasing number of hospital settings. Local experiences can provide valuable information pertaining to the reproducibility of this technique in different scenarios.
We performed a retrospective analysis of the clinical records of all patients that had undergone local anesthetic semi-flexible pleuroscopy in our unit between February 2015 and July 2017. Data on demographics, previous biochemical, cytological and histopathological analysis, procedure details, diagnostic and therapeutic results, complications and mortality were collected from all patients. Statistical analysis was performed using SPSS v23.
A total of 30 patients were included. They were mainly male (66.7%), with a median age of 72 years (minimum 19 years, maximum 87 years). All presented with exudative pleural effusions and the exam was performed for diagnostic reasons. Pleural tissue was obtained in all patients and the overall diagnostic accuracy was 93.3%. Malignancy was the chief group of diagnosis (66.7%), followed by pleural tuberculosis (13.3%). The procedure was well tolerated and self-limited subcutaneous emphysema was the only complication registered (13.3%). No deaths were associated with the procedure.
Our results globally overlap those of wider series and reinforce the perception that local anesthetic semi-flexible pleuroscopy is a well-tolerated, safe and highly accurate diagnostic and therapeutic tool which has proved to be both feasible and effective in our experience.
Ever since its introduction, over a century ago, by Jacobeus,1 pleuroscopy (also referred to as thoracoscopy or medical thoracoscopy) has progressed through the history of pulmonology with breakthroughs and drawbacks. After an initial period of enthusiastic utilization, the technique suffered a gradual decline in the second half of the 20 century not unconnected to the advent of effective antituberculosis therapy2,3 and perhaps heightened by the appearance of both blind pleural needle biopsy systems.4,5 Most centers, however, still retained the procedure, and by the end of the 20 century, a landmark paper6 established its operational characteristics which combined a high diagnostic yield (100% for pleural tuberculosis and 97% for malignancy) with a low profile of complications thus converting this procedure into the gold standard for pleural pathology.
Further technological developments, mainly driven by experimental reports on the utilization of sterilized fiberoptic bronchoscopes within the pleural cavity7,8 led to the introduction in 2002 of the semi-flexible pleuroscope (model XLTF-240; Olympus; Tokyo, Japan) – a device that allied a flexible 5cm length distal tip with a rigid 22cm length insertion portion connected to a proximal handle similar to that of a classic videobronchoscope.9
The difference in both equipment and biopsy forceps does not seem to significantly affect the diagnostic yield of semi-flexible pleuroscopy as recently observed in two independent randomized clinical trials comparing the classical rigid and the semi-flexible pleuroscope.10,11
A parallel issue that over the years has contributed to some degree of confusion is the different modalities of anesthesia and airway control used to perform this procedure. Some centers normally use general anesthesia and double lumen orotracheal intubation, while others perform this technique under spontaneous ventilation and conscious sedation in a modality most commonly designated as “local anesthetic thoracoscopy”.12
Combining the seemingly less invasive and more familiar handling of the semi-flexible pleuroscope with strategies of local anesthesia and spontaneous ventilation has the theoretical potential of advancing this procedure and making it more feasible in different hospital settings.
Our department is classified as level I (peripheral hospital) and currently covers a direct population of about 150,000 inhabitants. We introduced local anesthetic semi-flexible pleuroscopy in February 2015, and since December the same year we have also started to receive patients from two other nearby level I hospitals (increasing the coverage area to a total of about 300,000 inhabitants).
With this paper we aim to verify the feasibility and operational characteristics of this procedure and to compare our local experience with wider series in order to evaluate its reproducibility in different settings.
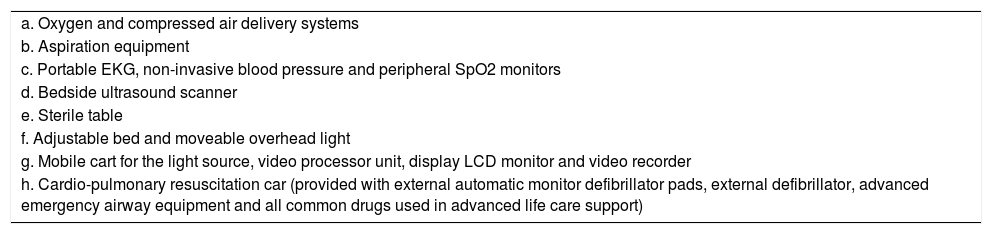
Material and methodsProcedure description – how we do itAs with previous descriptions13,14 all our procedures were performed in a clean endoscopy unit, equipped with all the supplies that are listed in Table 1. All our procedures were performed using the semi-flexible pleuroscope (model LTF-160; Olympus; Tokyo, Japan), under conscious sedation, local anesthesia and spontaneous ventilation. Based on international recommendations and current practice from different centers12–14 the procedure was conducted by one pulmonologist with the help of either a second pulmonologist or a pulmonology resident trainee who helped with instruments. A circulating nurse provided the required material for the different stages of the procedure. A second nurse was located at the bed head to monitor and control the level of conscious sedation and all the non-invasive cardio-pulmonary monitored parameters.
Equipment necessary for local anesthetic semi-flexible pleuroscopy.
| a. Oxygen and compressed air delivery systems |
| b. Aspiration equipment |
| c. Portable EKG, non-invasive blood pressure and peripheral SpO2 monitors |
| d. Bedside ultrasound scanner |
| e. Sterile table |
| f. Adjustable bed and moveable overhead light |
| g. Mobile cart for the light source, video processor unit, display LCD monitor and video recorder |
| h. Cardio-pulmonary resuscitation car (provided with external automatic monitor defibrillator pads, external defibrillator, advanced emergency airway equipment and all common drugs used in advanced life care support) |
Patients were carefully selected by previous clinical evaluation and the input from previous pleural fluid analysis, laboratory and imaging data. A written consent form was obtained for all patients.
Bedside focused lung ultrasound with a curvilinear 5Hz probe in B mode and abdominal pre-set was routinely performed before the procedure following a systematic protocol that has been described elsewhere.15 A slight adaptation of the standard protocol to include the additional ultrasound observation after the final positioning of the patient (in lateral decubitus) was added to help decide on the entrance site. Whenever possible the classical approach in the 5th to 6th mid-axillary intercostal space was chosen (modified only if required following the input from the ultrasound observation).
Following directions from international guidelines12 sedation was achieved with an initial bolus of 0.03–0.3mg/kg of intravenous midazolam followed by titrated intravenous midazolam (0.01–0.5mg/kg/h). Analgesia was obtained with intra-muscular 50–100mg single dose petidine, followed by intravenous tramadol (100mg).
After selection of the entrance point, all aseptic measures were conducted, and local anesthesia was applied with a subcutaneous lidocaine 2% solution. The entrance port usually required a skin incision of about 9–10mm from which the 8mm trocar could be inserted.
Once inside the pleural cavity all pleural fluid was aspirated and air was allowed to enter the pleural cavity in order to obtain full lung collapse which enabled a wider visualization of the pleural cavity.
From this point the pleuroscope was inserted and a systematic observation of the pleural cavity was performed in search for specific findings that could guide adjunctive procedures (pleural biopsies, adhesiolysis of simple pleural adhesions and talc poudrage instillation).
Pleural fluid was collected for biochemical, cytological and microbiological analysis. Pleural biopsies were routinely divided for microbiological and histopathological analysis. Light's criteria were used to classify the fluids as transudates or exsudates.16 The cut off value for positivity of pleural fluid adenosine deaminase (ADA) utilized by our laboratory was 40U/L.
Talc poudrage was performed with dry pressurized talc system (Steritalc spray, Novatech, SA) under direct optical guidance.
At the end of the procedure a chest drain (18–20F) was inserted and connected to an active aspiration system.
Data assemblage and statistical analysisAll data was retrospectively collected by consulting clinical file and included all patients who had undergone local anesthetic semi-flexible pleuroscopy between February 2015 and July 2017.
Data on demographics, previous biochemical and histopathological analysis, procedure details (including duration of chest tube drainage and overall length of hospital stay), diagnostic and therapeutic results, complications and mortality related to the procedure was collected.
Statistical analysis (descriptive statistic) was performed using the software SPSSv23 for all data collected and results are presented in terms of frequencies, percentages median and range when appropriate. Inferential analysis (regarding the comparison between sub-groups) was performed using independent samples t-test for normally distributed quantitative variables and U-Mann–Whitney test for non-normally distributed quantitative variables. Fisher's Exact Test was used to investigate the association between nominal variables. Normality of variables was evaluated with Shapiro–Wilk test. Results were considered as statistically significant if the p value was under 5%.
ResultsA total of 30 patients underwent this procedure during the study period. Patients were mainly male (66.7%), with a median age of 72 years (minimum 19 years, maximum 87 years).
All exams were performed for a diagnostic purpose, though 50% also performed talc pleurodesis at the end of the procedure.
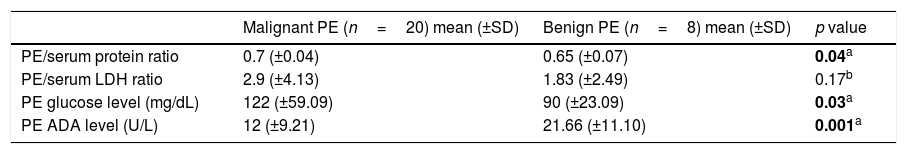
All pleural fluids were classified as exudates. When comparing the baseline ratio of pleural fluid to serum protein, lactate dehydrogenase (LDH), the pleural fluid glucose content and the pleural fluid level of adenosine deaminase (ADA) we found significant differences among the malignant and non-malignant pleural effusions (Table 2). Benign pleural effusions presented a significantly lower protein ratio, total glucose content and a significantly higher level of pleural fluid ADA. Lymphocyte predominance was observed in both groups (p value of 0.564 for Fisher's Exact test).
Biochemical characteristics of the pleural effusions.
| Malignant PE (n=20) mean (±SD) | Benign PE (n=8) mean (±SD) | p value | |
|---|---|---|---|
| PE/serum protein ratio | 0.7 (±0.04) | 0.65 (±0.07) | 0.04a |
| PE/serum LDH ratio | 2.9 (±4.13) | 1.83 (±2.49) | 0.17b |
| PE glucose level (mg/dL) | 122 (±59.09) | 90 (±23.09) | 0.03a |
| PE ADA level (U/L) | 12 (±9.21) | 21.66 (±11.10) | 0.001a |
PE=pleural effusion; LDH=lactate dehydrogenase; ADA=adenosine deaminase; SD=standard deviation.
Previous cytological results were positive for malignant cells in 30% of total (in which case the procedure was performed to collect tissue for histopathological and molecular analysis and simultaneous talc poudrage).
Bedside lung ultrasound revealed the presence of low to moderate septation in 20% of patients, and a modified intercostal approach was performed in 16.7%.
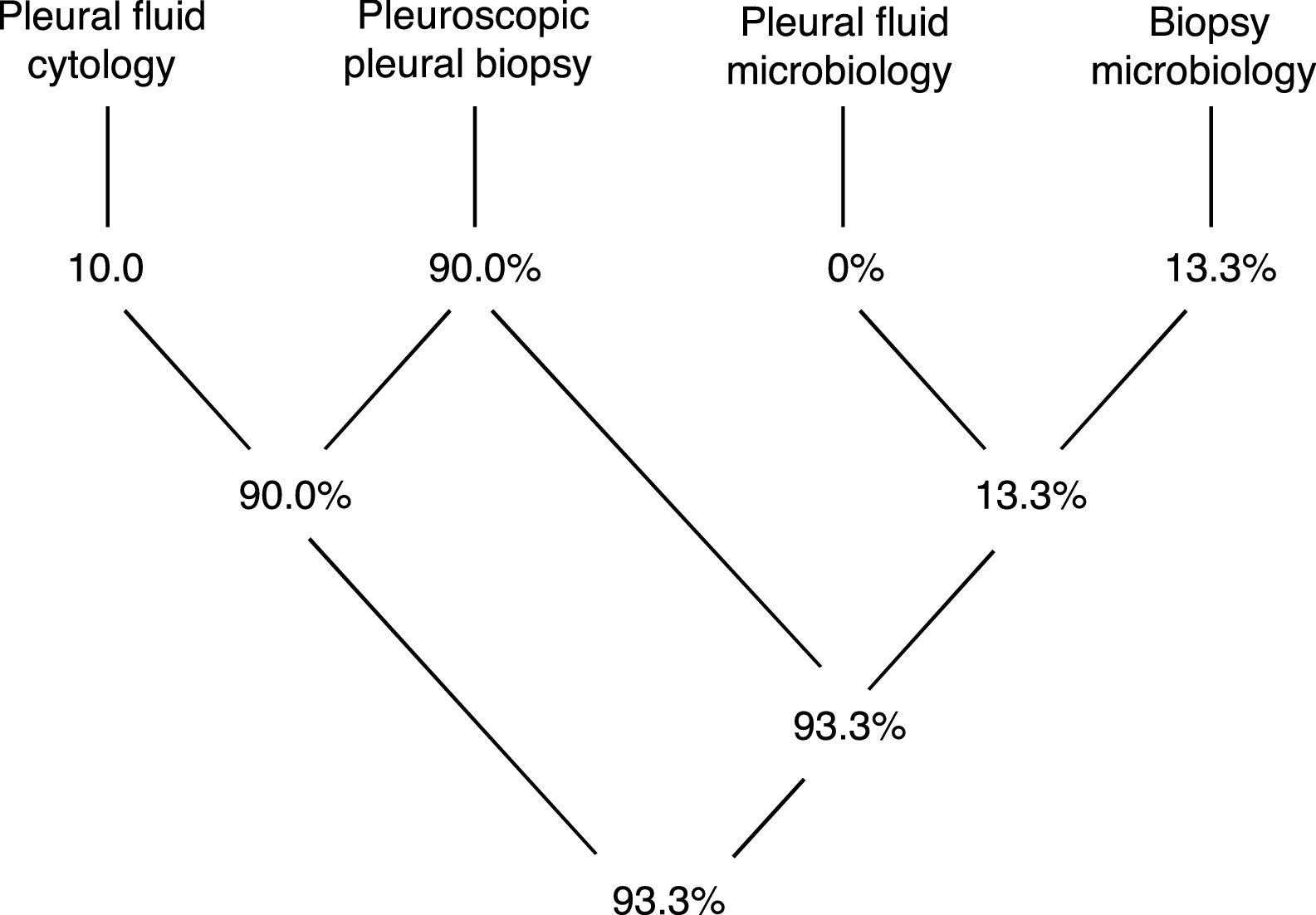
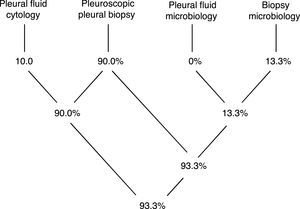
Pleural tissue was obtained in all procedures (technical yield of 100%), while a definitive diagnosis was obtained in 93.3% (Diagram 1).
The histopathological analysis provided a definitive diagnosis in 27 cases (90%) while one further case was diagnosed just through microbiology analysis of the biopsy specimen (3.3%). In 10% of cases there was an overlap of information gathered with cytology and histopathology, and in 10% there was overlap of information obtained with histopathology and microbiology (all referring to pleural tuberculosis). Only 2 cases (6.7%) remained undiagnosed with this procedure. Pleural biopsies of both these cases retrieved a histopathological diagnosis of non-specific pleuritis and further exams and follow-up strategies are still in place, but no alternative diagnosis has yet been obtained (both have follow up periods under 12 months).
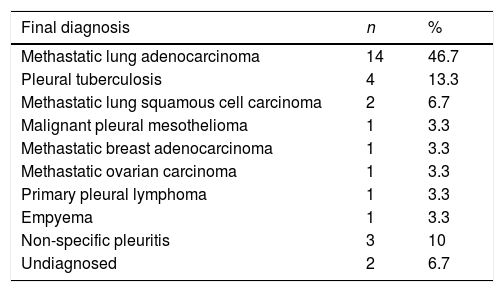
The complete list of final diagnosis observed is presented in Table 3. Malignant pleural effusions represented 66.7% of total. In all these cases the products obtained allowed a detailed immunohistochemical characterization. Molecular studies were required in 33.3% and in all cases the material collected was sufficient for this type of analysis.
List of diagnosis obtained.
| Final diagnosis | n | % |
|---|---|---|
| Methastatic lung adenocarcinoma | 14 | 46.7 |
| Pleural tuberculosis | 4 | 13.3 |
| Methastatic lung squamous cell carcinoma | 2 | 6.7 |
| Malignant pleural mesothelioma | 1 | 3.3 |
| Methastatic breast adenocarcinoma | 1 | 3.3 |
| Methastatic ovarian carcinoma | 1 | 3.3 |
| Primary pleural lymphoma | 1 | 3.3 |
| Empyema | 1 | 3.3 |
| Non-specific pleuritis | 3 | 10 |
| Undiagnosed | 2 | 6.7 |
Relating to microbiology, both pleural fluid and 2–3 biopsy specimens per patient were analyzed in search of common agents and Mycobacterium tuberculosis (Mt), but in our series only the pleural biopsy retrieved results. Direct exam (Zhiel–Nielsen stains) of pleural biopsy identified acid–fast bacilli in 2 cases and DNA for Mt was positive also in 2 patients. Positive cultures for Mt were obtained in 4 patients (13.3%). In all cases, drug sensitivity tests were performed and one case of isolated resistance to isoniazid was detected.
Further characterization (thoracic surgery) was required for one patient with pleuroscopic biopsies showing nonspecific pleuritis. The histopathological information retrieved from the surgical pleural biopsies was identical.
Pleurodesis was effective in 13 patients (86.6% of the total of patients submitted to talc instillation). In two cases post-pleuroscopy talc slurry was performed due to persistent pleural effusion drainage (exceeding 150mL/24h) 72h after the procedure.
Pleural drainage lasted a median of 4 days (range 2–15 days) and the full median in hospital length was 8 days (range 3–21 days).
The procedure was well tolerated under a median dose of 5mg of midazolam (range 2–12mg) and the standard described analgesia protocol. Self-limited subcutaneous emphysema was the only complication registered (13.3%). There were no further complications such as transient fever or add-on post procedure pain relief medication registered. One patient, diagnosed with lung cancer died 32 days after the procedure as a result of disease progression. No deaths were observed in relation to the procedure.
DiscussionOur results globally overlap those of larger series, namely in terms of diagnostic yield that has been reported to range from 78% to 97.4%.17–20
As with most other published data, malignancy was the biggest diagnosis group (representing 66.7% of total), while pleural tuberculosis (13.3%) was relatively low. This might reflect both a selection bias (patients with high pre-test probability for pleural tuberculosis were probably evaluated with alternative methods) but could also reflect our national and regional epidemiologic trends, since the global incidence of tuberculosis has been declining in Portugal,21 while the incidence of oncologic diseases has been gradually increasing, particularly in our specific region22 which has a strong association with demographic aging.
When exploring the differences in the biochemical characteristics of malignant and infectious pleural effusions, we found that significant differences were present in a few surrogate markers, most relevantly in the level of pleural fluid ADA which was found to be significantly higher in the non-malignant pleural effusion group. Nevertheless the pre-test probability for tuberculosis remained low in our sample given that all but one effusion had ADA levels below the threshold of 40U/L (the median value for all cases being 13U/L and ascending to 22U/L in the non-malignant group of effusions). This reinforces the much debated need for adjustments in the cut-off value for ADA depending on local epidemiological data,23,24 but of course larger samples are required to address this issue, preferably on a nationwide basis.
Current recommendations25,26 clearly state that immunohistochemical and molecular characterization of lung cancer is mandatory to achieve the best possible therapeutic approaches. This clearly requires physicians involved in the diagnosis of lung cancer to provide the pathologist with the best tissue specimens (both in quality and in quantity). In our sample, all malignant diagnosis were characterized with immunohistochemistry and molecular studies (for EGRF, ALK, ROS1 and PD-L1) were possible in all required cases, which means that all our procedures retrieved material of a quality and quantity considered sufficient for a detailed diagnosis of malignancy.
In relation to pleural tuberculosis all our diagnoses were based on culture positive biopsy results, while the pleural fluid microbiology was negative for all patients. This adds to the notion that pleural effusions in the context of pleural tuberculosis usually have a scarce and most often nonviable microbiological content24 and therefore tissue samples are a better way of achieving a diagnosis. The importance of culture based diagnosis relies not only on its high degree of certainty but also on the possibility of performing drug sensitivity tests (DST) that enable individualized therapeutic regimens when resistances are found, as was the case of one of our patients.
Nonspecific pleuritis, usually a diagnostic challenge, has been reported to range from 18% to 21%.18,19 In our series 5 patients (16.7%) received this histopathological information. Based on previous publications on this subject27 a follow-up strategy was completed for three patients where this was accepted as a final diagnosis since a possible cause could be ascertained (drug toxicity in one patient and metapneumonic pleural effusion in two patients). All three patients completed a 24 month follow-up without relapse. The remaining two patients are still considered undiagnosed and are currently under surveillance (at 6 and 8 months respectively). One of these patients was considered as high risk due to previous smoking history and sero-hematic macroscopic appearance of the pleural fluid so a more aggressive diagnostic strategy was conducted and the patient underwent thoracic surgery with repeated (and extended) pleural biopsies, but the diagnostic information remained the same. A PET-CT scan was negative and a close follow-up strategy is ongoing.
Though we cannot state that bedside lung ultrasound had a significant influence in our results we do believe it enhanced a safer approach to the pleural cavity since it allowed us to change the entrance site in a considerable number of patients to avoid adherences. Previously published data on this specific subject corroborates our results and favors the use of thoracic ultrasound prior to pleuroscopy,28,29 though the reported experience is scarce and operator dependent and randomized trials are desirable to definitely prove the add-on value of this technique.
In our series, the procedure was well tolerated with what we believe to be a relatively low dose of midazolam. From this perspective our results are comparable with previously published data30 and corroborate the proven safety profile (within the range of doses that were utilized) of midazolam for pleuroscopy.
Complications of the procedure were exceedingly low which we believe is due to careful patient selection, modality of sedation utilized, on-site thoracic ultrasound evaluation, and strict compliance to international recommendations and guidelines12–14 on absolute and relative contra-indications to the procedure. Also, diagnosis was the main focus of our procedures and only simple therapeutic measures (such as mechanical adhesiolysis of simple pleural adhesions and talc poudrage instillation) were performed, thus reducing the potential risk of complications.
Overall length of pleural drainage was similar to previously published data9 but total in-hospital length of stay is a newly published data on this subject that to the best of our knowledge has not been addressed by previous publications. The differences found were due to the fact that in three cases the procedure was performed on patients that had been previously admitted (with an average inpatient stay of 7 days) before being referred for the procedure. When adjusting these results by the date of the procedure and discharge date, a difference of only two days on average was found between pleural drainage and in-hospital stay. In all cases both these values were relatively low which in practice meant that an appropriate outpatient treatment could be offered to the vast majority of our patients. Further studies addressing this issue are required to validate our results.
ConclusionsLocal anesthetic semi-flexible pleuroscopy was a well-tolerated, safe and highly accurate diagnostic and therapeutic tool in our series. It proved to be both feasible and effective in our experience.
Conflicts of interestThe authors have no conflicts of interest to declare.