Endobronchial ultrasound-guided transbronchial needle biopsy (EBUS-TBNA) has proven to be an effective and minimally invasive tool to diagnose and stage lung cancer. However, its use for the diagnosis of rare mediastinal and lung pathologies has been rarely described. Hereby we describe a retrospective chart review of our EBUS-TBNA database for unusual diagnosis made between July 2012 and October 2016. Those conditions considered unusual for EBUS-TBNA diagnosis were identified and their medical records reviewed.
Endobronchial ultrasound-guided transbronchial needle biopsy (EBUS-TBNA) is a well-established tool for the diagnosis and staging of non-small cell lung cancer with reported sensitivity and specificity of 93% and 100% respectively.1,2 Recently, it has been increasingly recognized as a safe and minimally invasive tool for establishing the diagnosis of mediastinal and parenchymal masses of different etiologies, including various malignancies and rare benign diseases.3–5 The ability to perform adequate ancillary studies, such as immunohistochemical stains or molecular studies, on specimen obtained via EBUS-TBNA significantly enhances the specificity of the procedure, allowing pathologist to differentiate between different malignancies that might otherwise look similar cytologically. Furthermore, reports of genetic sequencing on specimen obtained by EBUS-TBNA have been reported in literature,6 and recently found to be comparable to histologic samples.7,8 Our manuscript describes the use of EBUS-TBNA for diagnosis of seven rare mediastinal and parenchymal pathologies with review of the current literature.
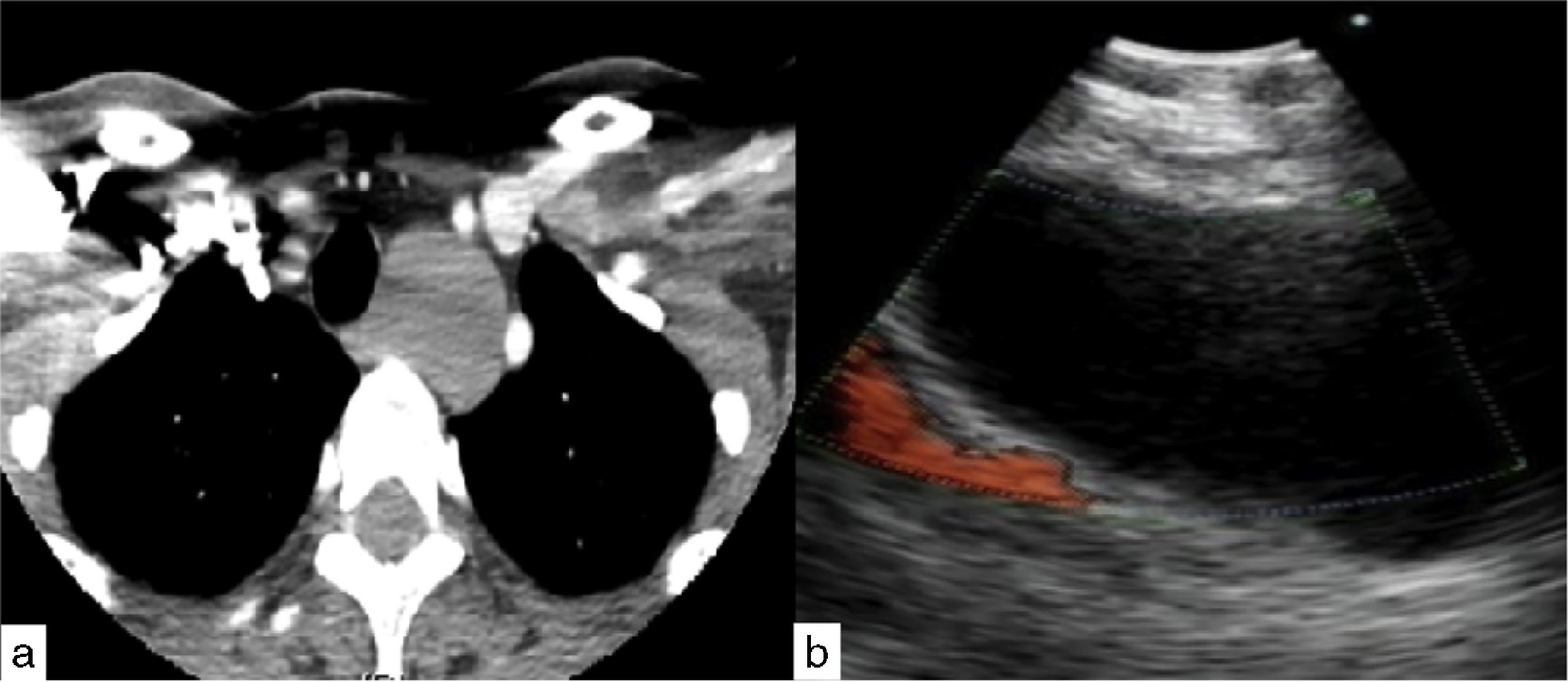
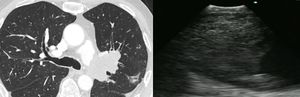
Case presentationCase 152 year-old woman presented with chronic non-productive cough and dysphagia to solids. Chest CT-scan revealed a 30×26mm left paratracheal mass amenable to EBUS-guided TBNA. An anechoic lesion was identified on endobronchial ultrasound, and transbronchial aspiration of 200ml of thick mucus using 21 Gauge needle was performed. The specimen was sent for cytology and microbiologic culture. Analysis revealed normal ciliated bronchial epithelial cells with no isolated organisms, thus compatible with benign uncomplicated bronchogenic cyst (Fig. 1).
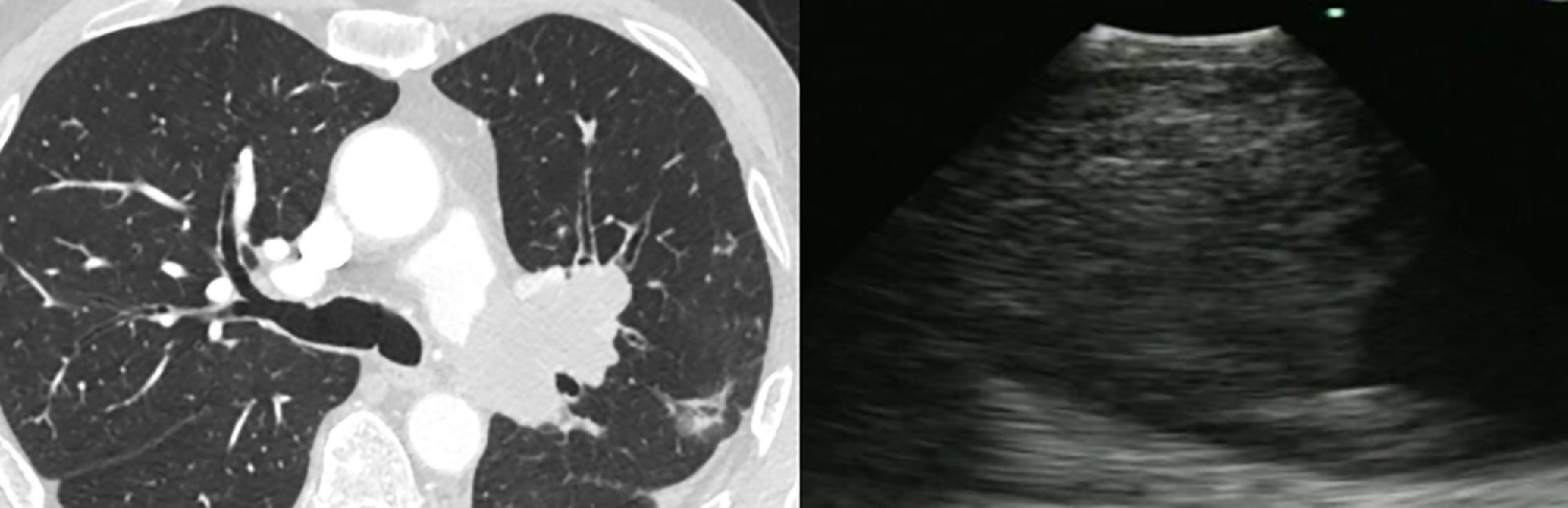
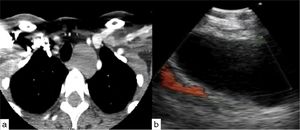
Case 2A 65 year-old man presented with chronic cough unresponsive to conventional therapy. A Chest CT scan revealed a lobulated low attenuation mass situated 8mm distal to the bifurcation of the left pulmonary artery measuring 45×62×33mm. He was referred for EBUS-TBNA. Cell block was positive for neoplastic cells, consistent with a spindle cell neoplasm with necrosis compatible with pulmonary artery sarcoma (Fig. 2).
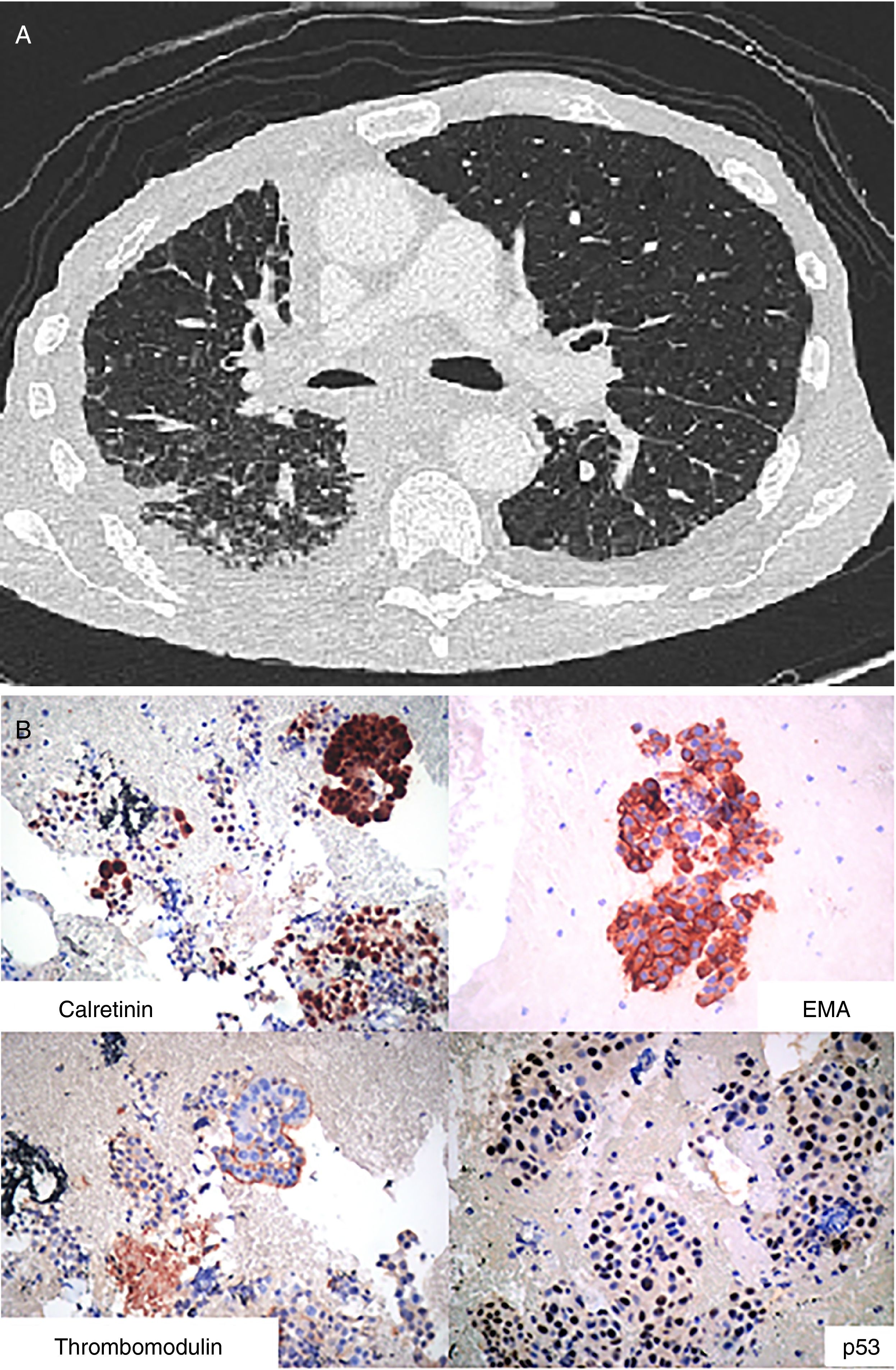
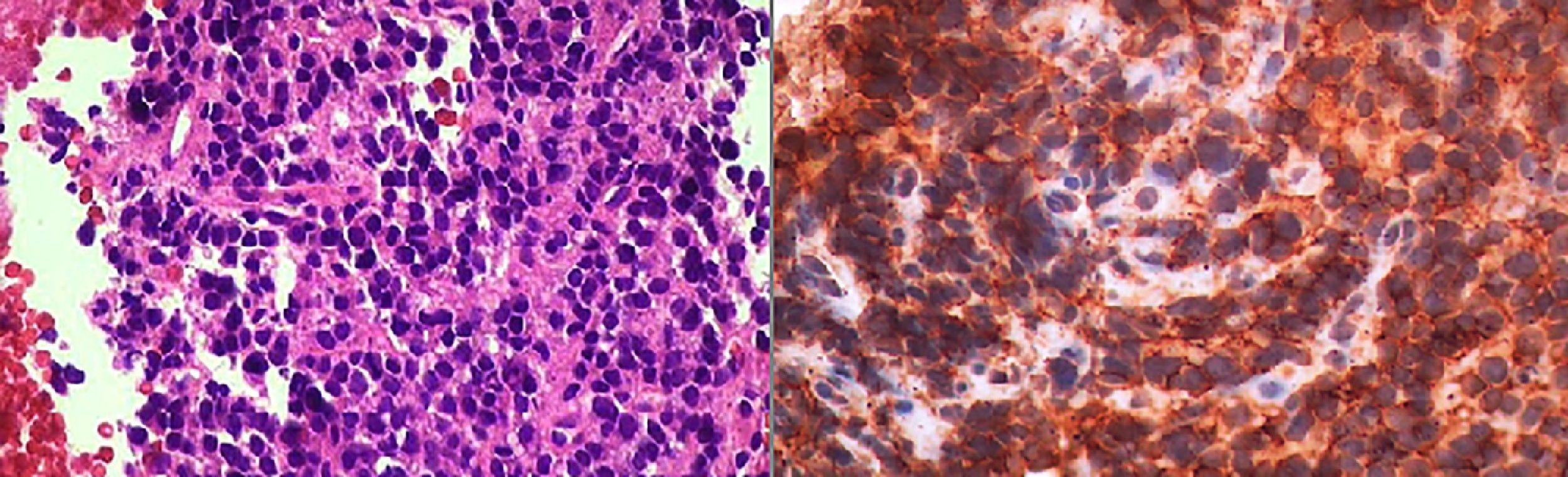
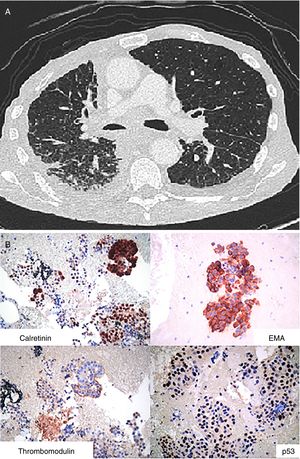
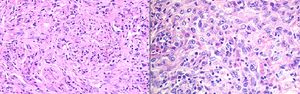
Case 3A 73 year-old man with a history of asbestos exposure, presented with recurrent right sided exudative pleural effusion. Pleural fluid cytology was highly suspicious for malignant mesothelial cells. PET-CT scan showed a diffuse patchy FDG-avid right pleural mass with associated mediastinal lymphadenopathy. The patient was referred to us for flexible broncoscopy and mediastinal staging. EBUS/TBNA of subcarinal lymph node revealed large epitheloid cells with moderate eosinophilic cytoplasm, central atypical nuclei, positive calretinin, thrombomodulin, and EMA, with negative CEA and TTF-1, consistent with malignant mesothelioma (Fig. 3).
Mesothelioma: (A) Axial image of CT scan of the chest showing diffuse thickening of the pleura and enlarged mediastinal lymphadenopathy. (B) Epithelioid cohesive cells with positive Calretinin and Thrombomodulin as well as p53 protein and EMA (epitelial membrane antigen) in a membranous distribution.
63 year-old woman referred for evaluation of a right peribronchial lesion seen on CT scan of the chest. Tissue obtained via EBUS-TBNA showed amorphous eosinophilic material with positive Red Congo stain. Polarized light microscopy showed yellow-green birefringence reflecting localized amyloidosis type AA (Fig. 4).
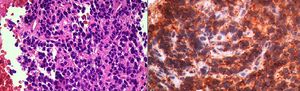
Case 525 year-old man with history of Ewing Sarcoma of the right femur resected 2 years prior, presented with a right interlobar lymph node positive on integrated positron emission tomography (PET-CT). EBUS-TBNA showed medium size cells with round nuclei, high nucleus-cytoplasm ratio, eosinophilic cytoplasm, CD99 positive, cytokeratin negative. These findings were consistent with mediastinal metastasis from Ewing Sarcoma (Fig. 5).
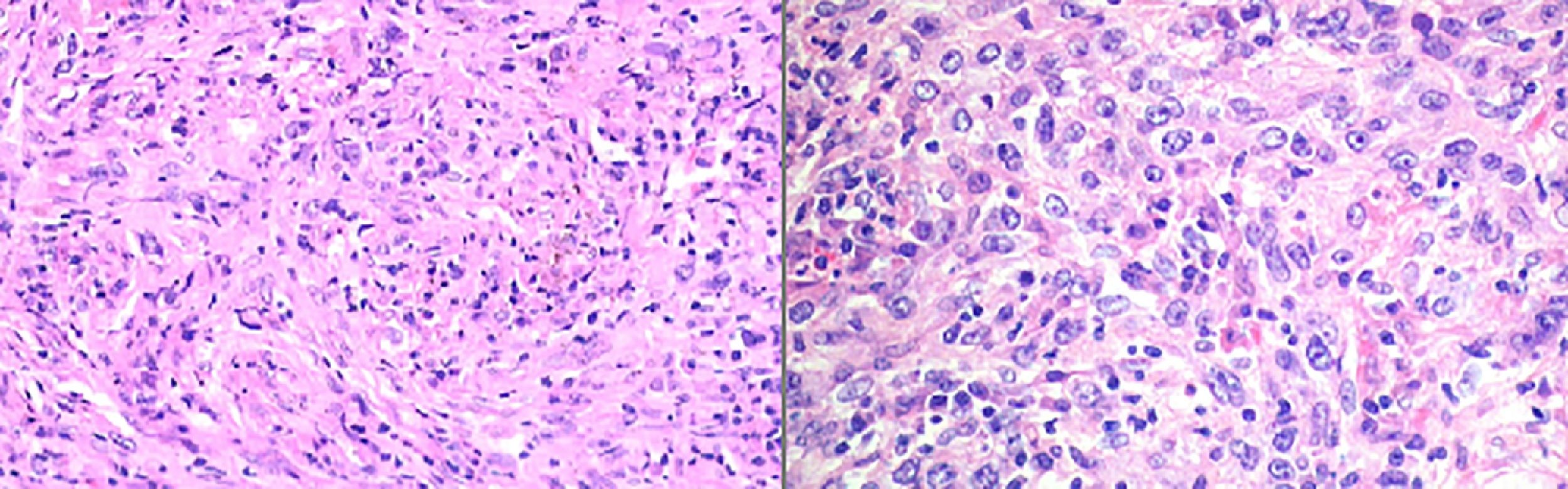
Case 657 year-old man with extensive smoking history presented for evaluation of chronic cough. Chest CT demonstrated a left upper lobe nodule, centrally located (16×18mm). This nodule was located adjacent to the left upper lobe take-off. EBUS-TBNA demonstrated fibrous tissue, fusoid and ovoid cells, nuclear pleomorphism and lymphoplasmocytic infiltrates. Since this was a high risk patient for malignancy, surgical resection was performed confirming the same findings and conclusive for the diagnosis of Inflammatory Pseudotumor (Fig. 6).
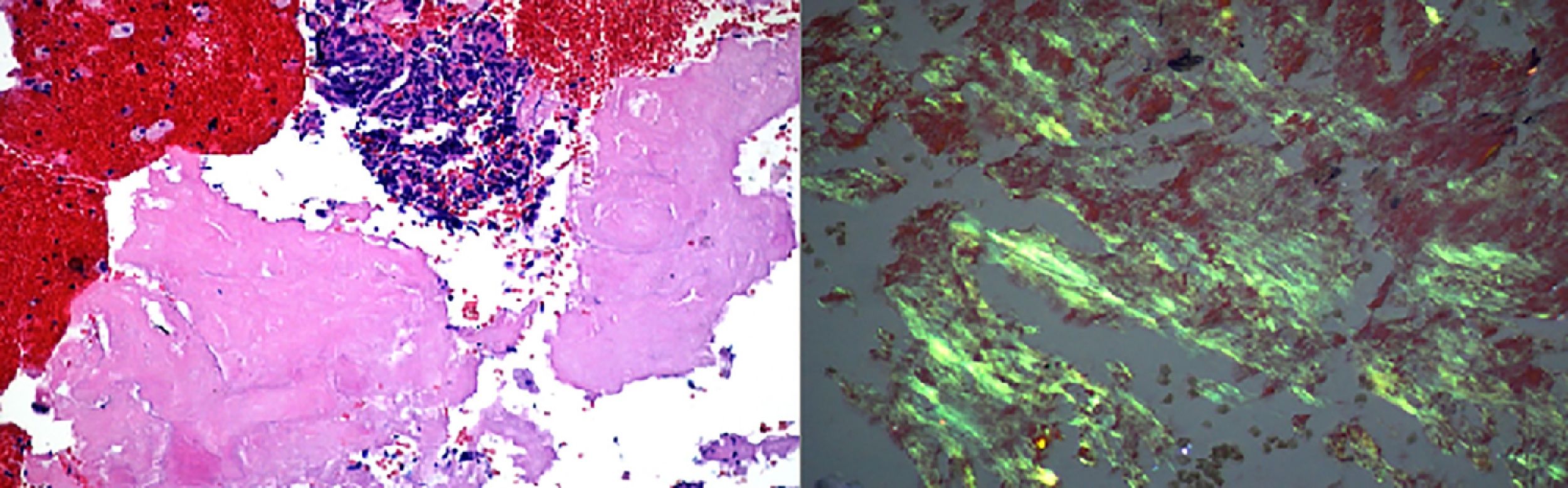
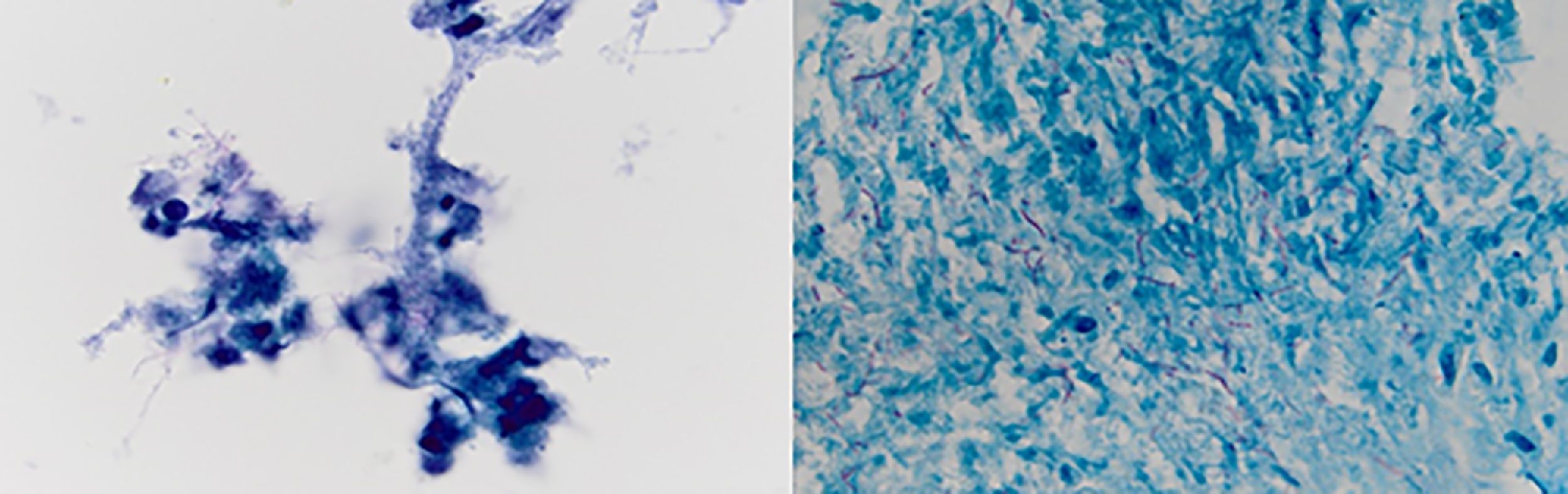
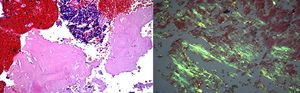
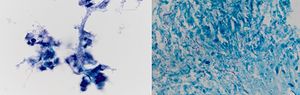
Case 760 years old woman with history of myelodysplastic syndrome status post bone marrow transplant from unrelated donor on immunosuppressive therapy, presented to the hospital with fever of unknown origin associated with chronic dry cough. Extensive infectious workup including lumbar puncture, blood and urine cultures were negative. CT scan of the chest showed a 4.5cm heterogenous mass with internal vascularity arising from the lingula, with associated mass effect at the junction of left main stem and superior segmental bronchi. This mass showed significant increase in size when compared to a CT scan done a month earlier. Flexible bronchoscopy with bronchoalveolar lavage, and EBUS-TBNA of the mass was performed. Gram stain of the aspirate showed gram positive branching filamentous organisms with cultures growing Nocardia farcinica (Fig. 7). Patient responded to antimicrobial therapy with significant reduction in size of the left hilar mass and improvement in symptoms.
Nocardia farcinica: (left) Papanicolaou stained ThinPrep slide demonstrating clusters of cellular inflammatory debris with associated filamentous microbacterial forms (1000× oil original magnification). (Right) A FITE stain (modified AFB stain) performed on the associated cell block material highlights the numerous variably branching filamentous bacteria, consistent with nocardia species (1000× oil original magnification).
EBUS-TBNA is currently the procedure of choice for diagnosis and staging of lung cancer.1 Being cost effective, less invasive with high diagnostic yield, allowing sampling of lymph node stations not accessible by mediastinoscopy, EBUS-TBNA has been increasingly used for diagnosis of mediastinal and parenchymal masses of various etiologies.4,5,9–12 A fair number of reported cases of rare pulmonary pathologies have been adequately diagnosed via EBUS-TBNA, otherwise sparing the patients invasive surgical approach.
The use of EBUS-TBNA has been described in the diagnosis and management of bronchogenic cysts.13 Although they are often asymptomatic, complications may arise including infection, bleeding, airway compression and rarely malignant transformation.14 Uncomplicated cysts are anechoic on ultrasound, and the presence of echogenic substances or septations may indicate complications such as infection or bleeding within the cysts.15 In addition, EBUS-TBNA can provide a reasonable alternative to excision for poor surgical candidates whose cyst is infected or large enough to create mechanical complications such as airway or esophageal compression.15–20 The use of real-time convex-probe ultrasound allows the complete aspiration of the cyst content under direct visualization, and consequently decreases the rate of recurrence by causing collapse and adherence of the cyst walls.18,19 Infection of the bronchogenic cyst following transbronchial aspiration has been reported, thus antibiotic prophylaxis is recommended.19–22
Aumiller et al. recently conducted a study which shed light on the use of EBUS for the diagnosis of central pulmonary embolism. This is particularly important in patient suspected to have vascular lesions such as angiosarcoma as a differential diagnosis.23 Certain features on CT scan such as extravascular spread of the mass, low attenuation filling defect, heterogeneous enhancement of mass occupying the entire lumen of central pulmonary artery may point toward a diagnosis of angiosarcoma.23 However, pathologic confirmation is necessary as the treatments differ substantially. EBUS not only help characterize the lesion but also to obtain tissue to confirm the diagnosis. Our case is the second reported on the use of EBUS for sampling and diagnosis of angiosarcoma. Park et al. published the first case, where the EBUS needle safely went through the pulmonary artery to sample the mass without complications.24
In the workup of mesothelioma, a detailed algorithm was published by Zielinski et al. in 2009, and the use of EBUS-TBNA to stage malignant pleural mesothelioma (MPM).25 According to this algorithm, patients with biopsy proven MPM, who are fit for surgery, should undergo EBUS-TBNA for mediastinal LN staging prior to surgery. Those patients with negative mediastinal lymph nodes should subsequently undergo laparoscopy to rule out intraperitoneal involvement. Most patients (77.7%) initially thought to be candidates for extensive multimodality approach including extrapleural pneumonectomy, were found to have distant metastasis using this algorithm, thus sparing them extensive unnecessary surgery.25
Leiro et al. who published the first case of the use of EBUS-TBNA needle for histopathologic diagnosis of mediastinal nodal amyloidosis.26 Primary pulmonary amyloidosis confined to the lung is very rare. It can present as tracheobronchial, interstitial, and nodular types.27 Pulmonary nodules can be single or multiple and may be hard to differentiate from primary or metastatic neoplasms.26,27 Needle aspiration show band-like hyalinized material which should test positive for Congo-red stain on pathology as shown in Fig. 5.26 A search of literature on Pubmed and Medline revealed one similar case, and other report using conventional TBNA needle for nodal sampling.28 Thus EBUS-TBNA is a helpful tool for initial diagnosis of pulmonary nodular amyloidosis however results should be interpreted with caution as amyloid could be associated with an underlying malignancy either admixed with the amyloid lesion or present at a distant site.28,29
Ewing sarcoma is high grade malignancy of major long bones which mainly affect children and young adults. Extraskeletal Ewing sarcoma (EES) arising in the mediastinum is extremely rare, and is an aggressive however potentially curable disease.30,31 Rare cases of pathologic diagnosis of EES using CT-guided fine needle aspiration of large mediastinal masses and other sites of metastasis or local recurrence, have been reported in literature.32,33 Our case is the first reported where EBUS-TBNA is successfully used for tissue sampling and cytopathologic diagnosis. Diagnosis of Ewing sarcoma is aided by with immunohistochemistry as cells will demonstrate positivity for MIC2 (CD99).30,34 We believe that EBUS-TBNA is not only safe and effective tool for tissue sampling but also allows concomitant staging of the mediastinum to rule out metastatic disease which will significantly affect prognosis. Ongoing studies are currently focusing on applying genetic studies on samples obtained by EBUS-TBNA which would further increase its specificity achieving diagnosis in rare histologic subtypes of tumors such as sarcomas.33
In addition, to the best of our knowledge this is the first case report of pulmonary inflammatory pseudotumor (IPT) diagnosed by EBUS-TBNA. Pulmonary Inflammatory pseudotumor is a very rare entity representing an inflammatory reaction in the lung or mediastinum.35–37 Given that IPT rarely transforms into malignancy and sometimes spontaneously regresses, asymptomatic lesions could be spared unnecessary surgeries.36 There are a few case reports published in the literature where CT guided transthoracic FNA and core biopsies are used to make the diagnosis.37 On cytopathology, it is characterized by spindle shaped cells on a background of inflammation. Those features are nonspecific and differential diagnosis is wide and includes melanoma, metastatic breast adenocarcinoma, sclerosing hemangioma, IgG4 sclerosing disease, and lymphoma to name a few, depending on the dominant cellular subtype.37,38 This explains why it is thought that the diagnosis of IPT should be by exclusion i.e. by ruling out other potential malignancies.38
Finally, EBUS-TBNA has also been shown to be a useful tool for diagnosis of infections such as mycobacterial tuberculosis,38 histoplasmosis, and blastomycosis. Pulmonary nocardiosis can present as nodules, lymphadenopathy, pleural effusions, or cavitations. Although the yield of transbronchial needle aspiration for nocardia has been previously reported to be low, Fujikura et al. published the first case report of nocardia diagnosed with EBUS-TBNA in an untreated HIV patient presenting with fever and mediastinal lymphadenopathy.39 To the best of our knowledge, we present the second case in English literature. Potential dissemination of the organism remains a concern given prior reports of mediastinitis in the setting of EBUS-TBNA,40 however this has not been reported. Minimizing the number of passes, performing it under real-time ultrasonography should minimize the risk.
ConclusionIn summary, EBUS-TBNA has been increasingly recognized as a safe and effective tool for establishing the diagnosis of mediastinal and pulmonary lesions.1 Enough tissue can be obtained to perform the additional testing (immunohistochemistry and fluorescent in situ hybridization) needed to make the diagnosis of rare entities such as the ones listed in this manuscript. In this way, patients are spared additional invasive procedures and unnecessary therapies. Larger studies are needed to assess the yield of EBUS-TBNA in establishing diagnosis, however, this could be challenging given the rarity of these diseases.
ContributionSF, AM: Provided the cases, wrote the manuscript, and approved the final proof.
HI: Wrote, submitted the manuscript, and approved the final proof.
IC, GL and MR: Reviewed, edited the manuscript, and approved the final proof.
Source of supportNo source(s) of support in the form of grants, gifts, equipment, and/or drugs are available for this manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.