In this issue of the Revista Portuguesa de Pneumologia – Portuguese Journal of Pulmonology Spinola and colleagues report about the predictors of mortality during tuberculosis (TB) treatment identified in Northern Portugal.1
Although the paper deals with a limited geographical area of a relatively small European country, the data reported raise some interesting issues for discussion. The study findings are of particular interest as Portugal remains a country with intermediate TB incidence rates (in a way close to those of Eastern Europe), but with an in-country variability depicting a country in-between persistence of intermediate TB incidence/transmission and shift towards low incidence/pre-Elimination dynamics.
Aim of the present manuscript is to discuss the importance of looking carefully at TB treatment outcomes, and in particular to TB mortality death rates, in the perspective of TB Elimination.
The findings of the study by Spinola et al.1 are discussed having in mind the treatment outcomes achieved in Europe and in the different WHO Regions. Data have been retrieved through the WHO and ECDC last annual reports and other relevant published figures and relevant documents.2, 3, 4, 5, 6, 7, 8
The World Health Organization (WHO) has recently launched the End TB Strategy,2, 3 which contains, among lines, several elements supporting TB elimination.
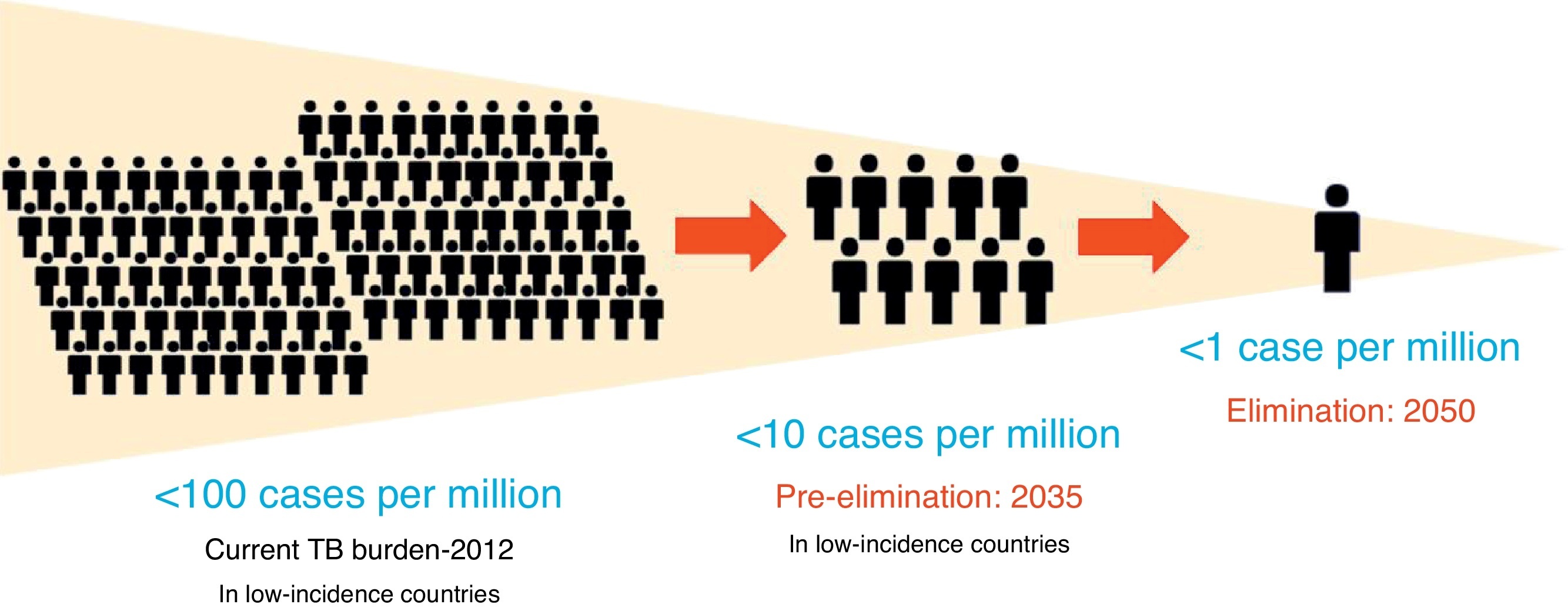
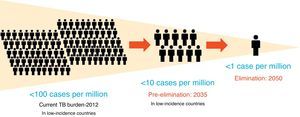
The definitions of TB elimination and pre-elimination are summarized in Figure 1.4
Figure 1. Definitions of elimination and pre-elimination.
The key elements to achieve TB elimination are represented by diagnosis and treatment of latent TB infection (LTBI), on top of the basic TB control interventions, which constituted the basis for the WHO DOTS strategy before and the Stop TB Strategy afterwards.5, 6, 7
Presently the End TB Strategy is composed of 3 pillars (Table 1). Pillar one includes the technical elements, diagnosis and treatment, and – for the first time – TB prevention, which are usually coordinated by a national TB programme (NTP) or equivalent body at the Ministry of Health level.
Table 1. End TB strategy.
| The End TB Strategy | ||||
| Vision | A world free of TB Zero TB deaths, Zero TB disease, and Zero TB suffering | |||
| Goal | End the Global TB epidemic (<10 cases per 100,000 population) | |||
| Indicators | Milestones | Targets | ||
| 2020 | 2025 | SDG 2030 | End TB 2035 | |
| Reduction in number of TB deaths compared with 2015 (%) | 35% | 75% | 90% | 95% |
| Reduction in TB incidence rate compared with 2015 (%) | 20% (<85/100,000) | 50% (<55/100,000) | 80% (<20/100,000) | 90% (<10/100,000) |
| TB-affected families facing catastrophic costs due to TB (%) | Zero | Zero | Zero | Zero |
| Pillars | Components | |||
| 1. Integrated, patient-centred care and prevention | A. Early diagnosis of tuberculosis including universal drug-susceptibility testing, and systematic screening of contacts and high-risk groups B. Treatment of all people with tuberculosis including drug-resistant tuberculosis, and patient support C. Collaborative tuberculosis/HIV activities, and management of co-morbidities D. Preventive treatment of persons at high risk, and vaccination against tuberculosis | |||
| 2. Bold policies and supportive systems | A. Political commitment with adequate resources for tuberculosis care and prevention B. Engagement of communities, civil society organizations, and public and private care providers C. Universal health coverage policy, and regulatory frameworks for case notification, vital registration, quality and rational use of medicines, and infection control D. Social protection, poverty alleviation and actions on other determinants of tuberculosis | |||
| 3. Intensified research and innovation | A. Discovery, development and rapid uptake of new tools, interventions and strategies B. Research to optimize implementation and impact, and promote innovations | |||
TB: tuberculosis; SDG: sustainable development goal.
TB prevention includes both vaccination (a new vaccine is expected by 2025) and the above mentioned diagnosis and treatment of LTBI.8
The second pillar includes all the different interventions that are necessary as a result of a coordinated action at different ministerial levels, as several ministries are likely to be involved: interior, finance, social affairs or equivalent, on top of ministry of health. Interventions like social protection, universal health coverage, or high quality vital registrations ideally require fine tuning of the legal framework by low TB incidence countries (initially called for action and defined as having a notification rate below 10 cases per 100,000 population, Table 1).
The third pillar emphasizes the importance of both developing new tools (prevention, diagnostic and treatment) and rationally introducing them at the programmatic level.9
It is clear that although difficult to achieve, TB elimination is epidemiologically plausible, as shown in the recently published WHO Elimination framework: all the low TB incidence countries are estimated to reach TB pre-elimination by 2035, as a preliminary step towards TB elimination, which is forecasted for 2050.4
Among the requirements of a good TB programme, and with the aim of controlling TB by early diagnosing infectious TB cases and rendering them rapidly non-infectious, case-finding and treatment outcomes are considered the core indicators and are constantly monitored.
The outcomes expected by a successful programme, together with those achieved in 2013 in the EU as well as in the different WHO Regions are summarized in Table 2.10
Table 2. Treatment outcomes for new and relapse cases, 2012 for the six WHO regions a and EU/EEA countries a .
| Outcome b | Theoretical good programme performances (%) | EU/EEA c (%) | WHO European d Region (%) | WHO African Region (%) | WHO/PAHO Region of the Americas (%) | WHO South-East Asia Region (%) | WHO Eastern Mediterranean Region (%) | WHO Western Pacific Region (%) |
| Treatment success | >85 | 77.1 | 75.51 | 81.31 | 74.61 | 87.81 | 87.31 | 92.21 |
| Death | <5 | 6.5 | 7.3 | 6.5 | 6.4 | 3.7 | 2.4 | 2.0 |
| Default | <5 | 4.8 | 6.2 | 5.7 | 7.7 | 5.1 | 4.6 | 1.5 |
| Failure | <2 | 0.9 | 5.1 | 0.9 | 0.8 | 1.2 | 0.8 | 0.5 |
a (WHO Regions: data extracted from WHO's global TB database. Available at: http://www.who.int/tb/country/data/download/en/ . Date last accessed: October 13, 2015. EU/EEA: data from European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2015. Stockholm: European Centre for Disease Prevention and Control, 2015. Available at: http://ecdc.europa.eu/en/publications/Publications/tuberculosis-surveillance-monitoring-Europe-2015.pdf ).
b New TB cases and relapse treatment outcome (%).
c EU/EEA (European Union/European Economic Area) comprises 31 Countries: 28 EU Member States (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom) and 3 EEA countries (Iceland, Liechtenstein and Norway).
d WHO European Region comprises the 53 countries: 28 EU Member States, 2 EEA countries (Liechtenstein not included), 23 non-EU/EEA countries (Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Georgia, Israel, Kazakhstan, Kyrgyzstan, the former Yugoslav Republic of Macedonia, Moldova, Monaco, Montenegro, Russia, San Marino, Serbia, Switzerland, Tajikistan, Turkey, Turkmenistan, Ukraine and Uzbekistan).
A core treatment outcome to be monitored is the death rate. Although this outcome has been initially conceived as death for any cause (TB being the most plausible cause, particularly in high TB incidence countries), the introduction of electronic, nominal databases has allowed to differentiate deaths for TB from deaths for other reasons.11
The paper by A.C. Franco Spinola et al. published in the present issue of the Journal1 merits special attention because of its choice of dealing with TB mortality in a region that can be considered a hot-spot for TB.
In particular, mortality during TB treatment in the evaluated cohort is in line with what reported in the EU/EEA countries and in WHO-EURO Region, i.e. 7.3% in this study vs. 6.5% and 7.3% in the two groups of countries (see Table 2 for details). This outcome is actually more in line with Eastern European NTP performances (all included in the WHO-EURO report) rather than Western European ones (only some Eastern countries are included in the EU/EEA report).10, 12
Notwithstanding that TB mortality is the hardest end-point of TB treatment, it has been till recent years a “neglected area” of interest in the evaluation of NTP performance or in the international TB literature, with the exception of settings with high HIV and/or MDR/XDR-TB prevalence. Actually, only in the last few years a few reports on predictors of TB mortality started to be published.13, 14, 15, 16, 17
Among the different TB treatment outcomes, mortality is the most important one to identify what went deadly wrong among patients enrolled in TB programmes. Identifying and evaluating patients’ characteristics associated to mortality during TB treatment may help to highlight the existing programmatic weaknesses where to invest for improvements.
In particular, the Authors of the report published in this issue of the Journal identified male TB patients over 45 years of age with prior cancer and with disseminated diseases as those at higher risk of death during TB treatment. This is in part similar, but not identical, to what reported by other Authors (Table 3).
Table 3. Predictors of mortality during tuberculosis (TB) treatment.
| Authors | Year of publication | Country | Predictors of mortality |
| Kourbatova et al. 13 | 2006 | Russia | Advanced TB; intravenous drug use |
| Lin et al. 14 | 2014 | Taiwan | Disseminated TB a ; malignancy a ; liver cirrhosis; renal failure |
| Alobu et al. 15 | 2014 | Nigeria | Extrapulmonary TB; smear-negative TB cases; rural residents; human immunodeficiency virus (HIV) co-infection; not receiving antiretroviral therapy; not receiving cotrimoxazole prophylaxis. |
| Pepper et al. 16 | 2015 | South Africa | Advancing age a , co-infection with HIV; prior history of TB; disseminated TB a |
| Liew et al. 17 | 2015 | Malaysia | Older age a ; male sex a ; foreign citizenship; lower Education; no bacille Calmette-Guérin (BCG) vaccination scar; rural residence; treatment in tertiary settings; HIV infection; not receiving directly observed treatment (DOT); advanced chest radiography findings; multidrug-resistant TB (MDR-TB); extra-pulmonary TB. |
a Same predictors of mortality as reported by Spinola et al. 1
In Spinola's paper1 lung cancer emerged as the most important co-morbidity associated to mortality. Such results may prompt initiatives to develop programmes of screening of those patients at higher mortality risk (e.g. lung cancer).
In different settings different predictors of mortality (see Table 3) can be observed during TB treatment, even within the same country. Nevertheless, once such predictors are identified, a strategy to confront with them need to be developed, tested and – if proven effective – implemented. Otherwise, the simple identification of mortality predictors would remain a mere “academic exercise” without a significant impact on programme performance and, most importantly, on TB patients’ health.
The important exercise of analysing systematically (and into details) the TB treatment outcomes (and, particularly, death rates), identifying programmatic drawbacks and tackling them with appropriate public health actions are part of the well-known TB managerial cycle.
If we want to push the paradigm shift from TB Control to Elimination forward, we need to start (or re-start) to perform systematically this as well as the other exercises aimed at monitoring case-finding and treatment results, which NTPs in several European countries have unfortunately started to forget.
Corresponding author. giovannibattista.migliori@fsm.it