The phenotypic variability in cystic fibrosis (CF) is widely recognized and modulated by environmental and genetic factors, including CFTR pathogenic variants and modifier genes genetic variants. In this context, determining the presence of variants in genes involved in immune response may allow a better understanding of CF variability, mainly in lung disease. Thus, ADIPOQ and STATH genes were selected and the analysis of exons and exon/intron junctions was performed for the determination of variations in its sequence, to determine the possible genetic modulation.
MethodsA total of 49 patients with CF, diagnosed for showing abnormal [chloride] levels in the sweat test, and identification of two pathogenic variants in CFTR categorized as class I and II were included. Genetic sequencing was performed for the identification of variants in the modifier genes.
ResultsIn our analysis, there was absence of rare genetic variants in STATH and ADIPOQ genes associated with the clinical variability. Thus, we are not able to establish an association between the disease severity and rare genetic variants in STATH and ADIPOQ genes, considering exons and exon/intron junctions.
ConclusionsConsidering the negative screening for rare genetic variants in ADIPOQ and STATH genes, it may be concluded that these genes are not associated with phenotypic modulation of CF in our population. To understand the modifier genes and its action at CF variability it is essential to promote a better overview of the disease. Also, negative reports can help to direct new studies without the use of unnecessary financial support.
The ADIPOQ (Adiponectin, C1Q And Collagen Domain Containing) is located in the 3q27 region, has three exons and encodes the adiponectin protein (GBP38, adipoQ, apM1 or Acpr30).1 Adiponectin belongs to the group of adipokines, which are produced by adipocytes, they are important in insulin sensitivity,2 energy metabolism and glucose sensitivity,3 vascular disease and immune response, acting as an anti-inflammatory factor.4,5 Adiponectin is expressed almost exclusively in adipose tissue, but a low expression occurs in other tissues.6,7 Also, adiponectin can modulate cytokine production in different types of myeloid cells and induce the production of the IL-10 mediator, having an anti-inflammatory and immunosuppressive effect in hematopoietic cells.8
Adiponectin occurs in its complete form as well as in fragments, and consists of the C-terminal globular, known as the globular domain of adiponectin. In its basic structure, adiponectin has 244 amino acids distributed in four domains: N-terminal sequence, variable domain, collagen domain and C-terminal globular domain.9 However, in its complete form, it can acquire different properties, such as monomers and trimers that can still associate with each other via collagen domains in groups of four to six, and thus result in oligomers with high molecular weight.7,10
Functional variants in ADIPOQ affect the levels of circulating adiponectin.11 These variants result in high levels of adiponectin in patients with CF and may modulate the disease phenotype by suppressing inflammation and improving the nutritional status.2 Corroborating the previous idea, it was shown that in the nasal epithelium of homozygous p.Phe508del, the most frequently expressed ADIPOQ occurred in mild lung disease.12
The STATH (Statherin) located in region 4q13.3 has five exons, which encode the statherin protein13 which is a peptide with antimicrobial properties expressed in the saliva, upper airways and nasal secretions, which participates in the development of biofilms of the oral cavity, mediating bacterial adhesion.2 Statherin is important in the saliva’s interaction with atmospheric air,12,14 having the role of maintaining oral health, working in conjunction with calcium and inhibiting crystal growth, showing high affinity for surfaces with hydroxyapatite. As a mediator of bacteria adhesion, statherin has epitopes that promote the growth and adhesion of certain microorganisms in the oral cavity (Porphyromonas gingivalis) while inhibiting the growth of others (Staphylococcus aureus).15 Although its antibacterial activity against Pseudomonas aeruginosa has not been investigated, it is known that infection with this bacterium accelerates the decline in the lung function.16 Also, statherin with high activity levels was found in homozygous p.Phe508del and with moderate and severe lung disease.12,17
Considering that the genetic profile of patients with CF for rare variants in ADIPOQ and STATH genes are still controversial and poorly understood, we aimed to identify sequence changes in exons and exon/intron junctions of ADIPOQ and STATH genes and to verify the existence of an association between its variants and CF severity.
MethodsParticipantsA total of 49 patients with CF diagnosed by (having shown [chloride] (≥60mEq/L)) the sweat test were included. All participants were homozygous or compound heterozygous for CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) pathogenic variants (class I or II). No patient was diagnosed via the neonatal screening test. The study was approved by the Institutional Ethics Committee of the University of Campinas (#570/2004). All participants or their parents signed a consent form before the beginning of the study.
DNA extractionThe DNA was obtained via phenol-chloroform extraction. The [DNA] used for analysis was 50ng/mL, evaluated using a GE NanoVue™ Spectrophotometer (GE Healthcare Biosciences, Pittsburgh, USA).
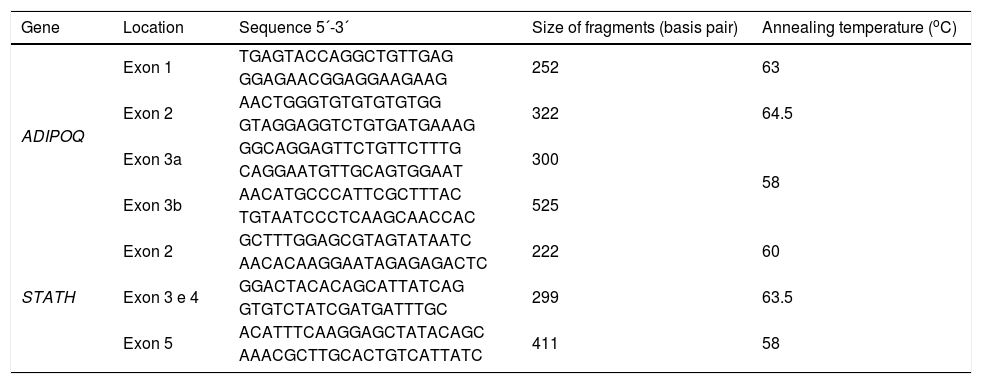
ADIPOQ and STATH screeningThe polymerase chain reaction (PCR) for amplification of ADIPOQ and STATH genes was performed with bidistilled water, 10× Taq buffer with (NH4)2SO4, MgCl2 (25mM), dNTP (25mM of each nitrogenous base), primers (0.2ρmol), Taq polymerase (5U) and genomic DNA (50ng/mL). The primers used in the analyses are shown in Table 1. The PCR conditions for ADIPOQ and STATH genes were 94°C/5min; 35 cycles of 94°C/1min, annealing temperature/1min and 72°C/2min; and 72°C/7min. The annealing temperature for each fragment analyzed is shown in Table 1.
Sequence of primers, size of the fragments and annealing temperature of ADIPOQ and STATH genes.
| Gene | Location | Sequence 5´-3´ | Size of fragments (basis pair) | Annealing temperature (oC) |
|---|---|---|---|---|
| ADIPOQ | Exon 1 | TGAGTACCAGGCTGTTGAG | 252 | 63 |
| GGAGAACGGAGGAAGAAG | ||||
| Exon 2 | AACTGGGTGTGTGTGTGG | 322 | 64.5 | |
| GTAGGAGGTCTGTGATGAAAG | ||||
| Exon 3a | GGCAGGAGTTCTGTTCTTTG | 300 | 58 | |
| CAGGAATGTTGCAGTGGAAT | ||||
| Exon 3b | AACATGCCCATTCGCTTTAC | 525 | ||
| TGTAATCCCTCAAGCAACCAC | ||||
| STATH | Exon 2 | GCTTTGGAGCGTAGTATAATC | 222 | 60 |
| AACACAAGGAATAGAGAGACTC | ||||
| Exon 3 e 4 | GGACTACACAGCATTATCAG | 299 | 63.5 | |
| GTGTCTATCGATGATTTGC | ||||
| Exon 5 | ACATTTCAAGGAGCTATACAGC | 411 | 58 | |
| AAACGCTTGCACTGTCATTATC |
ADIPOQ, Adiponectin; STATH, Statherin.
The sequencing of exons and exon/intron junctions of STATH and ADIPOQ genes was performed in MegaBACE® 1000 (GE Healthcare, Pittsburgh, PA, USA) using the DYEnamic ET Dye Terminator Cycle Sequencing Kit (with Thermo Sequenase™ II DNA Polymerase) (GE Healthcare, Pittsburgh, PA, USA) following the manufacturer’s recommendations. The sequences were analyzed using the Chromas Lite software, version 2.3.3.0 Chromas MFC application.18
Clinical markersThe clinical markers included were: CFTR pathogenic variants, age at the time of diagnosis, age at the onset of pulmonary and digestive symptoms, first infection by P. aeruginosa, spirometry, Shwachman-Kulczycki score, Kanga score, and transcutaneous oxygen saturation of hemoglobin.
For age at the time of diagnosis, age at the onset of pulmonary and gastrointestinal symptoms, and time until the first isolation of P. aeruginosa, the following groups were used: up to two months old, 13–36 months old, above 36 months old. In the case of digestive symptoms, meconium ileus was considered as an additional clinical marker. For P. aeruginosa, some patients were decolonized before the beginning of the study and were not included in the descriptive analysis.
Spirometric values were described considering forced expiratory volume in one second (FEV1%) as: (i) severe obstruction: <40%; (ii) moderate obstruction: ≥40% to <60%; (iii) mild obstruction: ≥60% to <80%; (iv) normal lung function: ≥80%.
Spirometry was performed using a speedometer CPFS/D model (Med Graphics, Saint Paul, Minnesota, USA). Data were recorded using the BREEZE PF software Version 3.8 B for Windows 95/98/NT.
The Shwachman-Kulczycki score was classified as excellent (86–100), good (71–85), mild (56–70), moderate (41–55) and severe (40 or less). The score was evaluated by two professionals, because it was a subjective analysis. In case of disparity between evaluators, a third evaluation was performed. The Kanga score was analyzed considering the presence or absence of exacerbation in the points obtained.
The transcutaneous oxygen saturation of hemoglobin was categorized as: (i) normal: ≥95%; (ii) mild hypoxemia: ≥91 to <95; (iii) moderate hypoxemia: ≥85 to <90; (iv) severe hypoxemia <85%.
ResultsPopulation analyzedOf the 49 patients with CF included in the study, 25/49 (51.02%) were female. Ages ranged from one to 26 years old, with the mean age being 9.71±6.06 years old. The mean age at the time of diagnosis was 29.70±2.47 months old. For the first P. aeruginosa, the mean age was 38.11±3.17 months old (Tables 2 and 3).
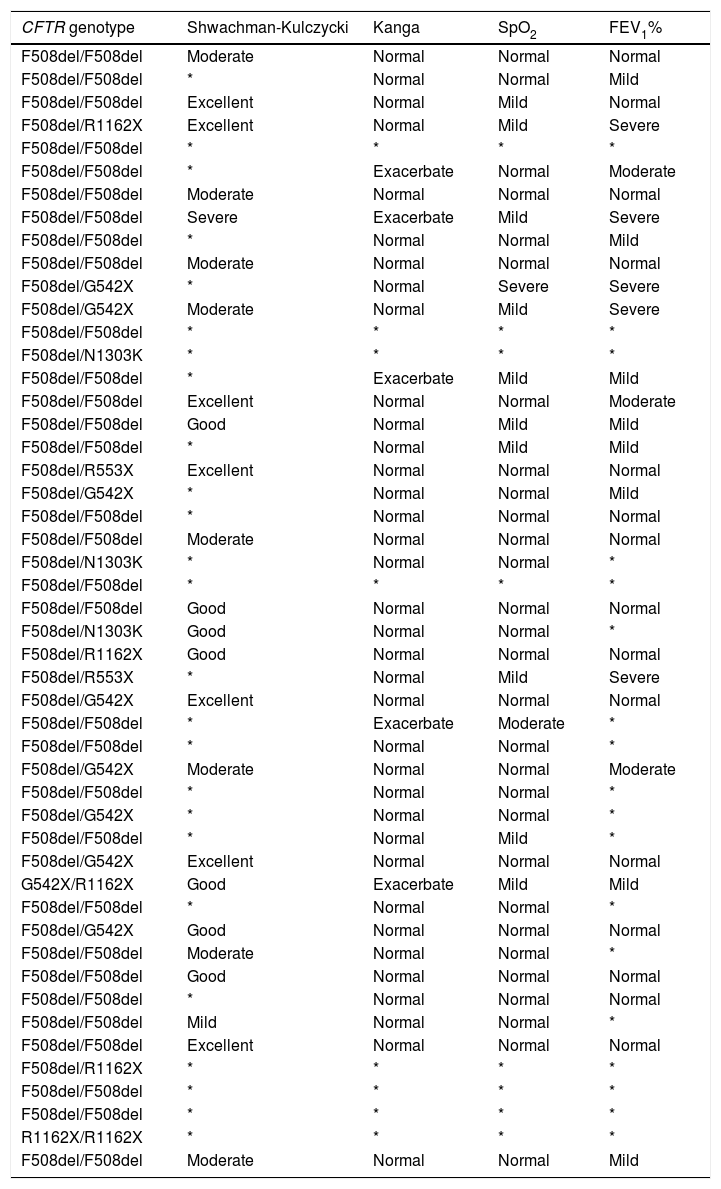
Characterization of the patients with cystic fibrosis for cystic fibrosis transmembrane regulator (CFTR) variants, Shwachman-Kulczycki score, Kanga score, transcutaneous oxygen saturation of hemoglobin (SpO2) and forced expiratory volume in the first second (FEV1%).
| CFTR genotype | Shwachman-Kulczycki | Kanga | SpO2 | FEV1% |
|---|---|---|---|---|
| F508del/F508del | Moderate | Normal | Normal | Normal |
| F508del/F508del | * | Normal | Normal | Mild |
| F508del/F508del | Excellent | Normal | Mild | Normal |
| F508del/R1162X | Excellent | Normal | Mild | Severe |
| F508del/F508del | * | * | * | * |
| F508del/F508del | * | Exacerbate | Normal | Moderate |
| F508del/F508del | Moderate | Normal | Normal | Normal |
| F508del/F508del | Severe | Exacerbate | Mild | Severe |
| F508del/F508del | * | Normal | Normal | Mild |
| F508del/F508del | Moderate | Normal | Normal | Normal |
| F508del/G542X | * | Normal | Severe | Severe |
| F508del/G542X | Moderate | Normal | Mild | Severe |
| F508del/F508del | * | * | * | * |
| F508del/N1303K | * | * | * | * |
| F508del/F508del | * | Exacerbate | Mild | Mild |
| F508del/F508del | Excellent | Normal | Normal | Moderate |
| F508del/F508del | Good | Normal | Mild | Mild |
| F508del/F508del | * | Normal | Mild | Mild |
| F508del/R553X | Excellent | Normal | Normal | Normal |
| F508del/G542X | * | Normal | Normal | Mild |
| F508del/F508del | * | Normal | Normal | Normal |
| F508del/F508del | Moderate | Normal | Normal | Normal |
| F508del/N1303K | * | Normal | Normal | * |
| F508del/F508del | * | * | * | * |
| F508del/F508del | Good | Normal | Normal | Normal |
| F508del/N1303K | Good | Normal | Normal | * |
| F508del/R1162X | Good | Normal | Normal | Normal |
| F508del/R553X | * | Normal | Mild | Severe |
| F508del/G542X | Excellent | Normal | Normal | Normal |
| F508del/F508del | * | Exacerbate | Moderate | * |
| F508del/F508del | * | Normal | Normal | * |
| F508del/G542X | Moderate | Normal | Normal | Moderate |
| F508del/F508del | * | Normal | Normal | * |
| F508del/G542X | * | Normal | Normal | * |
| F508del/F508del | * | Normal | Mild | * |
| F508del/G542X | Excellent | Normal | Normal | Normal |
| G542X/R1162X | Good | Exacerbate | Mild | Mild |
| F508del/F508del | * | Normal | Normal | * |
| F508del/G542X | Good | Normal | Normal | Normal |
| F508del/F508del | Moderate | Normal | Normal | * |
| F508del/F508del | Good | Normal | Normal | Normal |
| F508del/F508del | * | Normal | Normal | Normal |
| F508del/F508del | Mild | Normal | Normal | * |
| F508del/F508del | Excellent | Normal | Normal | Normal |
| F508del/R1162X | * | * | * | * |
| F508del/F508del | * | * | * | * |
| F508del/F508del | * | * | * | * |
| R1162X/R1162X | * | * | * | * |
| F508del/F508del | Moderate | Normal | Normal | Mild |
*, absence of data; F508del → c.1521_1523delCTT (p.Phe508del)], rs113993960; G542X → c.1624G>T (p.Gly542Ter), rs113993959; N1303K → c.3909C>G (p.Asn1303Lys), rs80034486; R553X → c.1657C>T (p.Arg553Ter), rs74597325; R1162X → c.3484C>T (p.Arg1162Ter), rs74767530.
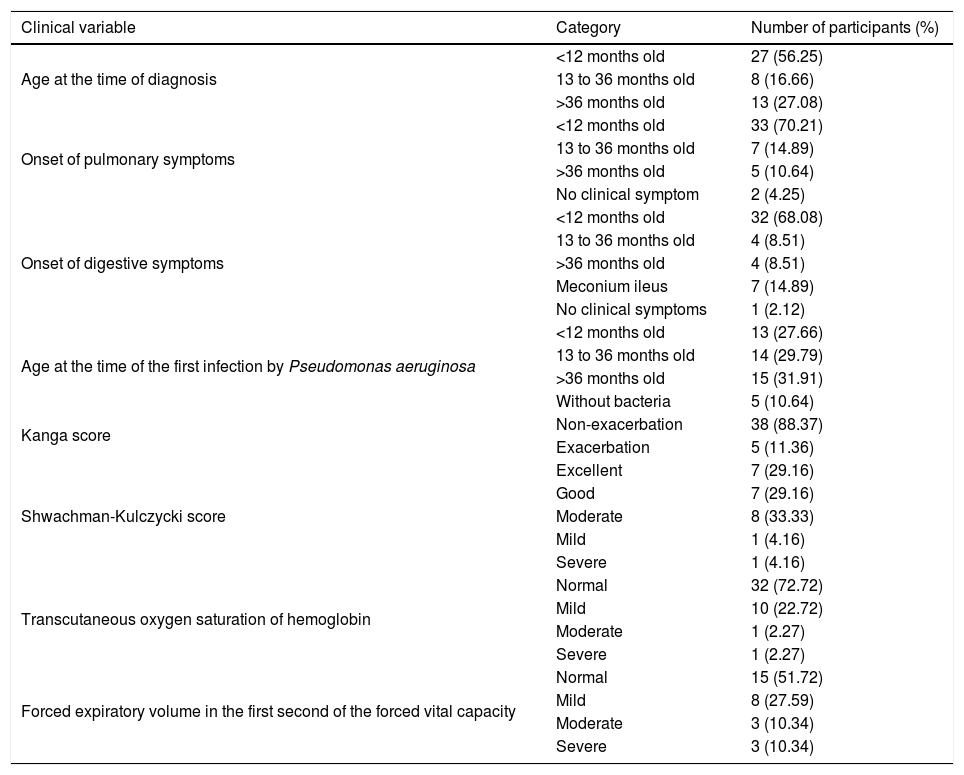
Distribution of the patients with cystic fibrosis according the clinical characteristics.
| Clinical variable | Category | Number of participants (%) |
|---|---|---|
| Age at the time of diagnosis | <12 months old | 27 (56.25) |
| 13 to 36 months old | 8 (16.66) | |
| >36 months old | 13 (27.08) | |
| Onset of pulmonary symptoms | <12 months old | 33 (70.21) |
| 13 to 36 months old | 7 (14.89) | |
| >36 months old | 5 (10.64) | |
| No clinical symptom | 2 (4.25) | |
| Onset of digestive symptoms | <12 months old | 32 (68.08) |
| 13 to 36 months old | 4 (8.51) | |
| >36 months old | 4 (8.51) | |
| Meconium ileus | 7 (14.89) | |
| No clinical symptoms | 1 (2.12) | |
| Age at the time of the first infection by Pseudomonas aeruginosa | <12 months old | 13 (27.66) |
| 13 to 36 months old | 14 (29.79) | |
| >36 months old | 15 (31.91) | |
| Without bacteria | 5 (10.64) | |
| Kanga score | Non-exacerbation | 38 (88.37) |
| Exacerbation | 5 (11.36) | |
| Shwachman-Kulczycki score | Excellent | 7 (29.16) |
| Good | 7 (29.16) | |
| Moderate | 8 (33.33) | |
| Mild | 1 (4.16) | |
| Severe | 1 (4.16) | |
| Transcutaneous oxygen saturation of hemoglobin | Normal | 32 (72.72) |
| Mild | 10 (22.72) | |
| Moderate | 1 (2.27) | |
| Severe | 1 (2.27) | |
| Forced expiratory volume in the first second of the forced vital capacity | Normal | 15 (51.72) |
| Mild | 8 (27.59) | |
| Moderate | 3 (10.34) | |
| Severe | 3 (10.34) |
In relation to the CFTR genotype, there was a high prevalence of homozygous p.Phe508del (63.26%). The p.Phe508del allele was the most prevalent (79.59%), followed by p.Gly542X (9.18%), p.Arg1162X (6.12%), p.Asn1303Lys (3.06%) and p.Arg553X (2.04%) (Table 2).
SequencingThe three exons of ADIPOQ gene and four of the five exons of STATH were analyzed. Exon 1 of the STATH gene was not analyzed being considered a non-translated region. The three and four exons were analyzed with a single primer pair. No changes (rare genetic variants) were found in all patients with CF for all fragments. Also, there is no need to compare among the homozygous subjects for CFTR and compound heterozygous subjects for CFTR because all CFTR variants included are severe variants.
DiscussionPopulation analyzedAccording to age range, most patients were included in the range between zero and ten years old (69.38%). Due to the inclusion of patients with pathogenic mutations in the class I and II CFTR group, these data may be associated with severe prognosis and low life expectancy considering the presence of severe mutations. However, the survival rates and prognosis of patients with CF have improved. One of the factors that has been associated with this fact is the systematic care of patients in specialized centers.19
Regarding sex, a uniform distribution was observed, a fact associated with autosomal recessive inheritance. However, the literature reports a slight predominance of males compared to females, increasing with the patients’ age.20,21 The lower prevalence in females may occur due to the vulnerability of females to certain clinical characteristics, such as the occurrence of diabetes mellitus.
The mean age at the time of diagnosis (2.47 years old) was higher than that found by Dorfman et al. (2008)22 (0.36 years old) in a group of 611 homozygous p.Phe508del. The average age at the time of the first infection by P. aeruginosa (3.17 years old) was lower than the one reported in the same study (7.5 years old). This difference can be explained by the lower age at the time of diagnosis of the previous study, compared to ours, suggesting that the early treatment of these patients can delay the colonization by P. aeruginosa.
ADIPOQLow adiponectin levels promote inflammation and are associated with increased insulin resistance and high risk of cardiovascular disease.23 Furthermore, adiponectin is associated with the regulation of energy balance, and in CF, chronic energy deficiency and thus higher [adiponectin] levels occur.24
Adiponectin has anti-inflammatory properties, mainly inhibiting the production of pro-inflammatory cytokines and inducting anti-inflammatory factors. However, higher levels of adiponectin were found in patients with CF compared to healthy subjects and as explanation, the presence of deficiency in the energy balance was considered24; moreover, the absence of correlation between inflammation markers [e.g. C-reactive protein (CRP) and fibrinogen] and adiponectin was reported, suggesting that adiponectin levels are not reduced in CF, even in the presence of low-grade inflammation or chronic infection/inflammation.23,25
In addition, functional variants in ADIPOQ have been associated with levels of circulating adiponectin.8,12 Some of these variants result in high levels of adiponectin, which could support a better clinical outcome of patients with CF, since adiponectin acts in suppressing inflammation-related diseases and improving nutritional status.2 In this context, two variants (exon 2 and 3) that decrease the level of circulating adiponectin were described.26 However, in our study, the presence of these variants was not supported.
In our study, it was not possible to establish the relationship between variants in ADIPOQ and CF severity, since no variants were found.
STATHStudies relating STATH and CF are scarce. However, it is known that statherin is an antimicrobial peptide expressed in the upper airways and nasal secretions involved in the development of biofilm in the oral cavity, mediating bacterial adhesion.2 It has recently been identified as the most prominent protein in the saliva’s interaction with atmospheric air.12,14
As a mediator of adhesion of bacteria, statherin has epitopes that promote the growth and adhesion of certain microorganisms in the oral cavity (P. gingivalis) while inhibiting the growth of others (S. aureus),15 although their antibacterial activity against P. aeruginosa has not yet been investigated. It is known that the infection by P. aeruginosa accelerates the decline in lung function.16 Thus, a protein that acts in bacterial adhesion in the oral cavity and upper airways is of extreme interest to studies related to the CF phenotype.
Statherin with high activity levels was found in homozygous p.Phe508del in moderate lung disease.12 This increase in expression was confirmed by an analysis of the mRNA produced by STATH in a sample of 12 patients with CF and moderate and severe pulmonary disease.17
In our study, it was not possible to establish a relation between variants in STATH and CF severity, since no variants were found.
ConclusionNo rare sequence alteration in the exon and exon/intron junctions of STATH and ADIPOQ genes were found. It was not possible to establish an association between CF and STATH and ADIPOQ genes for the regions analyzed in our study. It should be noted that the analyzed population is admixed and should have had greater polymorphic variability than other previously studied populations, which did not occur in our data.
Conflict of interestsThe authors declare no conflict of interests.
Authors’ contributionCAACC/FALM/JDR/CSB contributed to the study’s conception and design, acquired, analyzed and interpreted the data, drafted the manuscript and revised its intellectual contents, and approved the manuscript for publication.
We thank Luciana Cardoso Bonadia, Taís Daiene Russo Hortencio, Kátia Cristina Alberto Aguiar, Aline Cristina Gonçalves and Antônio Fernando Ribeiro for assistance in data collection and organization of ideas, Maria Angela Ribeiro for spirometry analysis.
[FALM] thanks the São Paulo Research Foundation (FAPESP, acronym in portuguese) for sponsoring #2011/12939-4, #2011/18845-1, #2015/12183-8 and #2015/12858-5; the Research, Teaching and Extension Support Fund (FAEPEX, acronym in portuguese) of the University of Campinas for sponsoring #0648/2015; [JDR] thanks FAPESP for sponsoring #2011/18845-1 and #2015/12183-8.