Cardiac magnetic resonance (CMR) imaging has gained importance in pulmonary hypertension (PH) and studies have demonstrated its use as a surrogate marker and in following treatment of these patients. The pathophysiology of PH differs between pulmonary arterial hypertension (PAH, group 1) and chronic thromboembolic PH (CTEPH, group 4).
ObjectivesThe present study tested the hypothesis that PAH and CTEPH display different characteristics on CMR imaging.
Methods46 patients were evaluated for pulmonary vascular disease in the French National Reference Center for PH (23 PAH and 23 CTEPH matched for age and gender). All patients had the right heart catheterization (RHC) and CMR imaging performed within 48h. CMR imaging was performed on a 1.5 T scanner.
ResultsPAH and CTEPH had similar body surface area and similar invasive hemodynamics, including mean pulmonary arterial pressure, cardiac index, pulmonary vascular resistance and right atrial pressure. PAH and CTEPH had similar CMR data. Right ventricular (RV) morphology and function and pulmonary artery (PA) data were also similar.
ConclusionAge- and sex-matched PAH and CTEPH patients displayed similar values of the CMR indices of RV and PA morphology and function, suggesting that the RV-PA responses are similar in both groups, mostly related to the overall increase in after load.
Cardiac magnetic resonance (CMR) imaging has gained significant importance for the evaluation of pulmonary hypertension (PH) patients1 in recent years, not only as the gold standard tool for the evaluation of right ventricle (RV) but as a significant surrogate marker in the follow-up of these patients.2 Although many of the RV remodeling features seen in PH have been well described in CMR studies,3 little is known about the differences in CMR indexes between different PH etiologies.
The pathophysiology of PH differs significantly between pulmonary arterial hypertension (PAH, group 1) and chronic thromboembolic PH (CTEPH, group 4). The PAH group includes patients with precapillary PH with similar pulmonary angioproliferative vasculopathy that predominantly affects the precapillary arterioles.4 CTEPH occurs when there is obstruction of the pulmonary vascular bed by nonresolving thromboemboli, differing from PAH because of its major vessel involvement.5 How much these different pulmonary vascular involvements lead to distinct RV remodeling processes is something not clearly demonstrated. The present study aimed to compare the RV features at CMR in PAH and CTEPH.
MethodsForty-six patients evaluated for pulmonary vascular disease in the French National Reference Center for PH entered in the study. The data collection was part of a standardized diagnostic approach registry set up in agreement with the Commission Nationale de l’Informatique et des Libertés, the organization dedicated to information technology and civil rights in France. The study enrolled 23 PAH and 23 CTEPH patients matched for age and gender evaluated during diagnostic approach. Of the 23 patients with CTEPH, 13 had proximal disease and were eligible for surgery and 10 patients had contraindications fore surgery due to predominant distal disease.
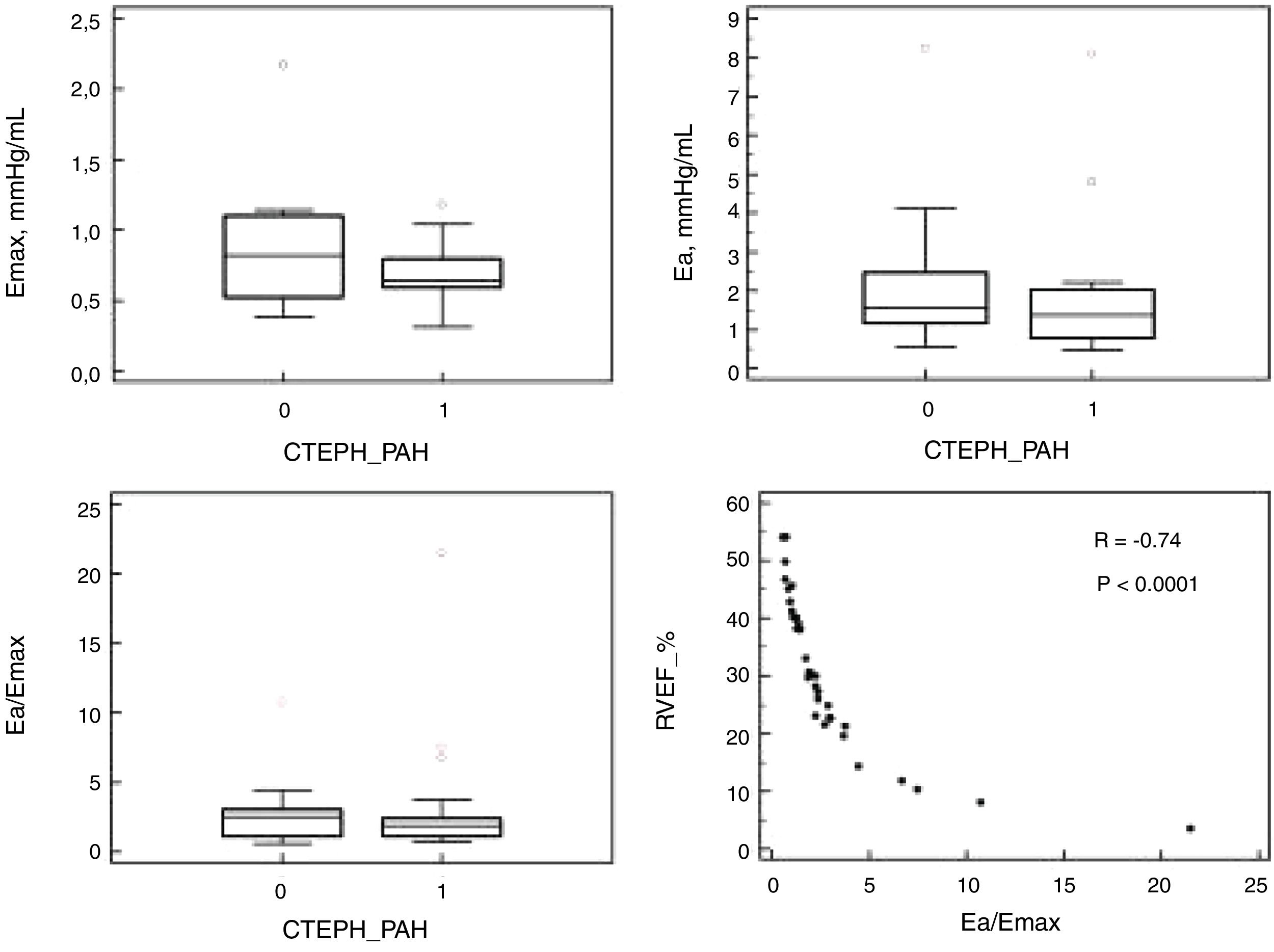
All the patients had the right heart catheterization (RHC) and CMR imaging within 48h. All RHC measurements followed the protocol previously described.3 CMR imaging was done on a 1.5T scanner with electrocardiographic gating (Siemens, Germany). Ventricles volumes and areas and PA measurements followed the protocol previously described.3 In the pulmonary artery (PA) trunk cine images, PA areas were manually delineated at maximal and minimal dilation (MaxPA/MinPA) for the calculation of PA pulsatility: PApulsatility=100×(MaxPA−MinPA)/MinPA. Phase-contrast sequence was used for the determination of flow velocity in the PA trunk. The PA effective elastance (Ea, index of arterial load) and the RV maximal end-systolic elastance (Emax, index of contractility) were calculated for 33 patients (14 CTEPH, 19 PAH). The ratio Ea/Emax was calculated to study the RV-PA coupling: Ea=(mPAP−PAoP)/SV and Emax=mPAP/ESV (SV=RV stroke volume; ESV=RV end-systolic volume, both measured by CMR).6
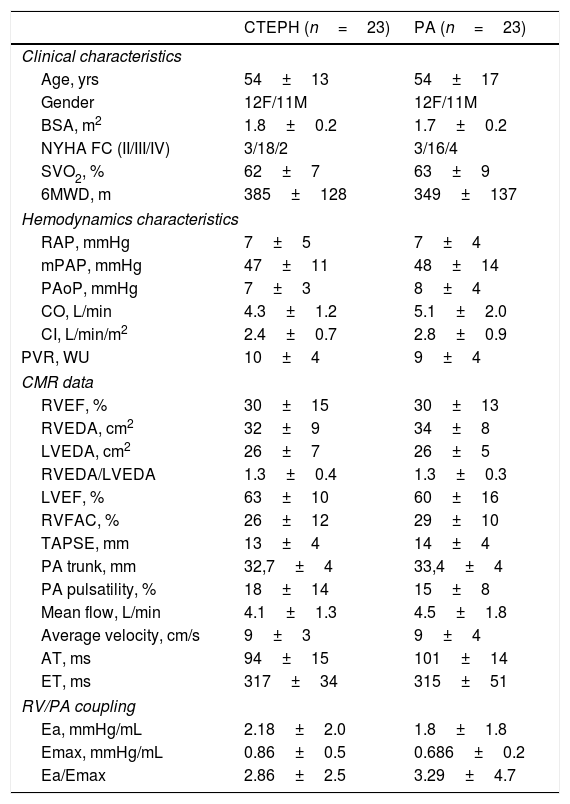
ResultsPAH and CTEPH patients had similar anthropometric and functional characteristics, including body surface area, functional class and 6-min walking distance. Furthermore, hemodynamic severity was also similar in both groups, including mean PA pressure, cardiac index, pulmonary vascular resistance and right atrial pressure. On CMR, ventricular morphology and function did not differ between groups, as the pulmonary artery characteristics and indexes of RV/PA coupling (Table 1 and Fig. 1).
Clinical and hemodynamic characteristics and CMR data.
| CTEPH (n=23) | PA (n=23) | |
|---|---|---|
| Clinical characteristics | ||
| Age, yrs | 54±13 | 54±17 |
| Gender | 12F/11M | 12F/11M |
| BSA, m2 | 1.8±0.2 | 1.7±0.2 |
| NYHA FC (II/III/IV) | 3/18/2 | 3/16/4 |
| SVO2, % | 62±7 | 63±9 |
| 6MWD, m | 385±128 | 349±137 |
| Hemodynamics characteristics | ||
| RAP, mmHg | 7±5 | 7±4 |
| mPAP, mmHg | 47±11 | 48±14 |
| PAoP, mmHg | 7±3 | 8±4 |
| CO, L/min | 4.3±1.2 | 5.1±2.0 |
| CI, L/min/m2 | 2.4±0.7 | 2.8±0.9 |
| PVR, WU | 10±4 | 9±4 |
| CMR data | ||
| RVEF, % | 30±15 | 30±13 |
| RVEDA, cm2 | 32±9 | 34±8 |
| LVEDA, cm2 | 26±7 | 26±5 |
| RVEDA/LVEDA | 1.3±0.4 | 1.3±0.3 |
| LVEF, % | 63±10 | 60±16 |
| RVFAC, % | 26±12 | 29±10 |
| TAPSE, mm | 13±4 | 14±4 |
| PA trunk, mm | 32,7±4 | 33,4±4 |
| PA pulsatility, % | 18±14 | 15±8 |
| Mean flow, L/min | 4.1±1.3 | 4.5±1.8 |
| Average velocity, cm/s | 9±3 | 9±4 |
| AT, ms | 94±15 | 101±14 |
| ET, ms | 317±34 | 315±51 |
| RV/PA coupling | ||
| Ea, mmHg/mL | 2.18±2.0 | 1.8±1.8 |
| Emax, mmHg/mL | 0.86±0.5 | 0.686±0.2 |
| Ea/Emax | 2.86±2.5 | 3.29±4.7 |
BSA: body surface area, NYHA: New York Heart Association functional class, SVO2: mixed venous saturation, 6MWD: six-minute walking distance, RAP: right atrium pressure, mPAP: mean pulmonary artery pressure, PAoP: pulmonary capillary artery occluded pressure, CO: cardiac output, CI: cardiac index, PVR: pulmonary vascular resistance, RVEF: right ventricle ejection fraction, RVEDA: right ventricle end-diastolic area, LVEDA: left ventricle end-diastolic area, LVEF: left ventricle ejection fraction, RVFAC: RV fractional area change, TAPSE: tricuspid annular plane systolic excursion,PA trunk: pulmonary artery trunk diameter, AT: acceleration time, ET: ejection time, Ea: effective elastance, Emax: RV maximal end-systolic elastance. All comparisons between the two groups with p>0.05.
Our study tested the hypothesis that CMR data could differ from IPAH and CTEPH since the pathophysiology and outcome of these two forms of PH are different. Nevertheless, comparing two groups with similar functional and hemodynamic features, we found no differences regarding RV remodeling or PA at CMR, despite the clear distinctions in vascular involvement in PAH and CTEPH.
PAH and CTEPH have different pathophysiology, prognosis and management. Patients with PAH should be treated with specific medication and patients with CTEPH should be evaluated for pulmonary thromboendarterectomy (PEA).5 CMR studies have demonstrated the significant difference in CMR indexes in patients with CTEPH before and after PEA. After PEA, RV dimensions normalized, RV volumes decreased significantly and hypertrophy of the RV decreased; improvement of RV and LV function is also observed after surgery. In PAH, changes in CMR reflecting improvements in left and right ventricular function with the use of targeted therapies7 mirrored changes in functional capacity, hemodynamic severity and survival.2,8 These findings suggest that CMR similarly correlates with disease progression and severity in both clinical conditions, comparable to our results.
The RV generates a pulsatile flow and the PA has the role of dampening RV flow pulsatility. With the changes in elastic properties of the PA seen in PH, there is an increase in stiffness of the PA and consequently changes in this RV-PA coupling. Sanz et al. demonstrated alterations in PA elasticity early in the course of the disease. Patients with normal values of mPAP at rest with increase with exercise had elevated stiffness index when compared to controls.9 Since CTEPH compromises more proximally the PA, the RV-PA coupling impairment and its consequences in RV remodeling and PA alterations could be different from PAH. Nevertheless, our study suggests that both groups have similar RV response to increased afterload at rest as we found similar indexes of right ventricular/PA coupling.
The main limitation of our study is the small sample size. We decided to match both groups according to gender and age to minimize the impact of these characteristics in the comparison between the two study groups. Another limitation is that we did not perform measurements during exercise which prevented extrapolation of our results. Furthermore, patients had comparable hemodynamic profile, reinforcing our findings regarding the similarity in the RV response facing an increased afterload, even as a consequence of two distinct pathophysiological mechanisms. PA characteristics were also similar in both groups despite the fact that CTEPH has a major vessel involvement. Altogether, these findings suggest that the RV response is mainly a consequence of the overall imposed afterload, instead of a predominance of proximal or distal disease.10
ConclusionOur study demonstrated that age- and gender-matched PAH and CTEPH patients displayed similar values of the CMR indices of RV and PA morphology and function, suggesting that the RV response is similar in both groups, mostly related to the overall increase in after load.
Conflicts of interestThe authors have no conflicts of interest to declare.