Screening for latent tuberculosis infection (LTBI) in close contacts of infectious TB cases might include Tuberculin Skin Test (TST) and Interferon-Gamma Release Assays (IGRA), in combination or as single-tests. In Portugal, the screening strategy changed from TST followed by IGRA to IGRA-only testing in 2016. Our objective was to compare the cost-effectiveness of two-step TST/IGRA with the current IGRA-only screening strategy in immunocompetent individuals exposed to individuals with respiratory TB.
Materials and MethodsWe reviewed clinical records of individuals exposed to infectious TB cases diagnosed in 2015 and 2016, in two TB outpatient centers in the district of Porto. We estimated medical, non-medical and indirect costs for each screening strategy, taking into account costs of tests and health care personnel, travel distance from place of residence to screening site and employment status. We calculated the incremental cost-effectiveness ratio (ICER) as the cost difference between the two screening strategies with the difference number of LTBI diagnosis as a measure of cost-effectiveness, assuming that treating LTBI is a cost-effective intervention. We also calculated adjusted odds-ratios to test the association between diagnosis of LTBI and screening strategy and estimated the total cost for averting a potential TB case.
ResultsWe compared 499 contacts TST/IGRA screened with 547 IGRA-only. IGRA-only strategy yielded a higher screening effectiveness for diagnosing latent tuberculosis infection (aOR 2.12, 95%CI: 1.53 - 2.94). ICER was €106 per LTBI diagnosis, representing increased effectiveness with a slightly increased cost of IGRA-only screening strategy.
ConclusionsOur data suggests that in Portugal LTBI screening with IGRA-only is more cost-effective than the two-step TST/IGRA testing strategy, preventing a higher number of cases of TB cases.
Systematic diagnosis and treatment of latent tuberculosis infection (LTBI) is a key part of the TB elimination strategy in low-incidence countries.1 Screening strategies in individuals with close contact with infectious cases of TB include the tuberculin skin test (TST) followed by the interferon-gamma-release assay (IGRA) in individuals with positive TST results (two-step strategy); single-step IGRA testing; and single-step TST testing.2
IGRA tests have specificity greater than 95% in the diagnosis of LTBI.3 The TST specificity is similar (97%) in populations not vaccinated with Bacillus Calmette-Guérin (BCG), but is considerably lower (60%) in those vaccinated.3 Sensitivity of the two tests is roughly the same: 80-90% for IGRA; 80% for TST.3,4 Some studies have shown that the progression rate (likelihood that a person with a positive test will develop active TB) is higher in IGRA-positive individuals.5,6,7
A cost-effectiveness study in the United Kingdom estimated that two-step TST/IGRA screening strategy is less costly than single-step IGRA testing (£162,387 vs. £ 203,983 per 1000 contacts).2 However, this study only considered medical costs. In France, a decision analysis model, considering only direct medical costs, showed that in almost all scenarios QuantiFERON (QFT) was more effective and cost-effective than TST in detecting LTBI.8
One study in Brazil, though, showed that the most cost-effective strategy was TST (US$ 16,021/averted case) and that the incremental cost-effectiveness ratio was US$ 227,977/averted TB case for QFT-GIT.9 Another study of individuals entering the Dallas County Jail (Texas, United States) reported a substantially higher positivity rate of IGRA than TST: these authors suggested that sensitivity of TST screening was lower, and that IGRA was more time-efficient and associated with four-fold lower indirect costs. The overall cost per LTBI case detected was nearly three-times higher for the TST than the IGRA.10
A recent report of the European Centre for Disease Prevention and Control used a deterministic TB transmission model to predict the impact of different LTBI screening and treatment strategies for several risk-groups, including contacts of TB cases. They concluded that from the healthcare perspective, LTBI screening is most cost-effective when done using the two-step approach (TST first and, if positive, followed by IGRA).11,12
Given the heterogeneous results of different studies, the World Health Organization advised more research in this field.13
The Portuguese National Health Service maintains TB outpatient centers that are responsible for TB and ITBL diagnosis, treatment, and screening across the country, under technical guidance from the National Tuberculosis Programme. In the Northern Region, TB outpatient centers switched from the two-step TST/IGRA to the single-step IGRA-only screening strategy, after shortages in tuberculin supply. Before this switch, TST was performed to exposed contacts immediately after diagnosis of TB in index case and repeated 8-10 weeks after the last exposure of risk, followed by IGRA testing (QuantiFERON Gold Plus ®) whenever TST was positive. After 2016, IGRA-only (QuantiFERON Gold Plus ®) was performed only once, 8-10 weeks after the last exposure to index case of TB. The IGRA assay available during the study period in the Northern Region was the QFT-Plus assay.14,15
The objective of this study was to compare the cost-effectiveness of the two strategies described above, in terms of medical costs (for the healthcare system) and direct and indirect non-medical costs related to LTBI screening (excluding treatment costs), for LTBI screening in close contacts of confirmed cases of respiratory TB.
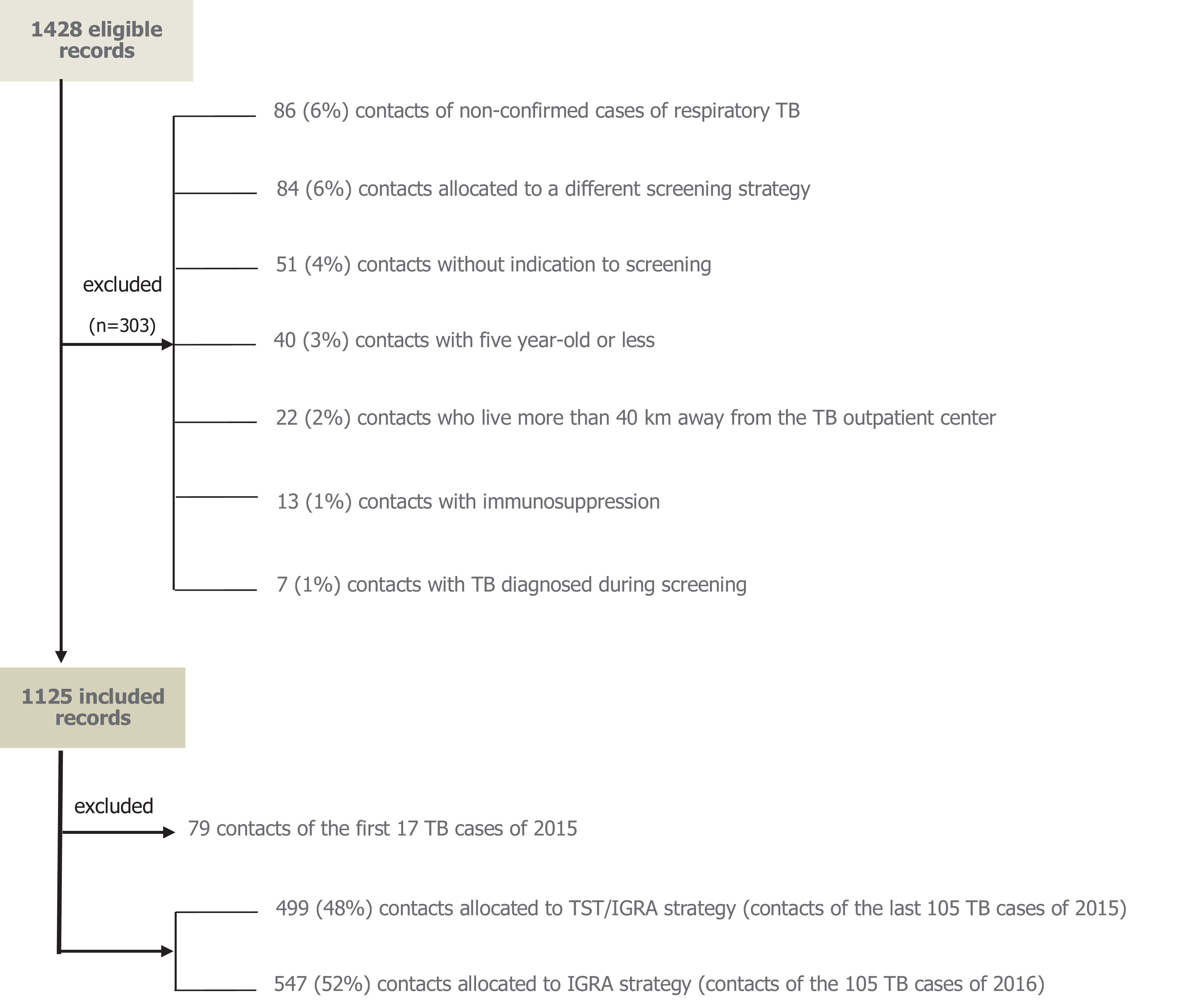
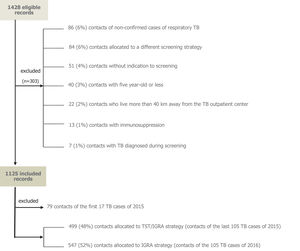
Material and MethodsPatient SelectionWe examined clinical records of all individuals screened for TB and LTBI in two TB outpatient centres from the Northern Health Region: Penafiel (180,000 inhabitants, annual TB incidence rate of 47.7/105 in 2012-16) and Vila Nova de Gaia (330,000 inhabitants, annual TB incidence rate of 26.0/105 in 2012-16).16 Only individuals exposed to an infectious TB patient, diagnosed from 1 January 2015 to 31 December 2016, were included. Exclusion criteria were those described in Figure 1.
We extracted socio-demographic information (sex, age, parish of residence, employment status), clinical information (history of TB/LTBI, immunosuppression, diagnosis of TB during screening), LTBI screening test information (screening strategy, date[s], result[s], diagnosis, initiation of treatment), and information on the index TB cases (diagnosis date, sputum smear results).
Cost EffectivenessCost-effectiveness was expressed as an incremental cost-effectiveness ratio (ICER), which was calculated by dividing the cost difference between the two screening strategies with the difference number of LTBI diagnosis.11,17 We assumed that treating LTBI is a cost-effective intervention and focused only in the differences between strategies until the moment of LTBI diagnosis.
The median costs in the TST/IGRA and TST-only groups were compared using Mann-Whitney test. The proportion of individuals diagnosed with LTBI, number of visits to the TB outpatient center, and adherence to screening were also compared for these two groups using the Chi-squared test or Fisher's exact test, as appropriate. Adjusted odds-ratios (aORs) and 95% confidence intervals (95% CIs) for each screening strategy and diagnosis of LTBI were calculated using logistic regression. Screening strategy, sex, age, place of residence, professional status (employed/unemployed), TB outpatient center, index case sputum smear positivity, infectious period, and site of disease in the index case were included in the initial regression models. In this procedure, a backward stepwise approach was used, and at each step, the least significant variable that was not a substantial confounder (whose removal would lead to a change of more than 20% in the OR of one or more parameters remaining in the model) was removed. Sex and index case sputum smear positivity were retained regardless of p-value.
Direct individual medical costs were calculated as the sum of estimated costs from the screening test(s), specimen transportation to the testing laboratory, and the work of healthcare professionals (collection and testing of specimens) – data provided by the Northern Regional Health Administration for 2014-2016. IGRA (QuantiFERON-TB Gold Plus) cost was €37.66 (including ELISA kit, antigen, and mitogen) and the TST cost was €1.00 (including tuberculin). Disposable material costs were estimated using online prices (including laboratory materials, blood collection materials, tuberculin needles and syringes, gloves, compresses), and were calculated as €0.31 for one TST and €0.57 for one IGRA.
Direct non-medical costs were estimated per screened individual by multiplying the number of visits to the health center by the distance traveled (calculated taking into account patient's address), with an estimated cost of €0.10 per km. To assess the impact of this estimate on final results, a sensitivity analysis was also performed using an estimated cost of €0.35 per km, the reference value used by the Portuguese Government. This cost was adjusted considering that contacts of the same index case could share their mean of transport if they went to the TB outpatient center on the same day.
Indirect costs per screened individual were calculated by multiplying the number of visits to the health center by the half-daily average income (from Instituto Nacional de Estatística, Statistics Portugal) for working individuals. Half-daily income was used in order to not overestimate indirect costs, considering that TB is associated with socio-economic deprivation.18,19 A sensitivity analysis was also performed using daily average income. The number of potentially averted TB cases was estimated based on the number of individuals who started LTBI treatment, a 10% lifetime risk of developing TB, and a 70% efficacy of treatment (assuming that all patients who began LTBI treatment finished their treatment).20
DefinitionsThe following definitions were used in this study:
- •
Adherence to screening: proportion of individuals who showed up for screening that completed all recommended screening steps;21
- •
Confirmed respiratory TB patient: person with a positive respiratory TB diagnosis (tracheal, laryngeal, bronchial, pulmonary, and/or pleural), confirmed by a positive culture for Mycobacterium tuberculosis complex (MTC) or a positive smear plus MTC nucleic acid detection in sputum or bronchoalveolar lavage;22
- •
Close contact: person who had close contact with a patient who had respiratory TB for a cumulative time of at least 8 h if sputum smear-positive, or 40 h if sputum smear-negative (National Tuberculosis Programme recommendation);23
- •
Immunosuppressed individual: individual receiving chemotherapy, radiotherapy, an immunosuppressive drug, or infected with HIV;21
- •
Period of infectiousness: time interval during which MTC may be transferred between individuals, estimated as the number of days between onset of symptoms and TB diagnosis;24
- •
Latent tuberculosis infection: positive IGRA test in an individual who does not have active TB;24
- •
Medical direct costs: healthcare-related costs (screening tests, specimen transportation to the testing laboratory, and work of healthcare professional);21
- •
Non-medical direct costs: costs related to the transportation of an individual to a TB outpatient center for screening;21
- •
Indirect costs: productivity loss from an individual's absence from work because of travel to the TB outpatient center for screening.21
Stata® IC 15.0 (Student version) was used for statistical analysis. Ethical approval for this study was obtained from the Ethics Boards of the Institute of Public Health of the University of Porto (CE17061) and the Northern Regional Health Administration (39/2017).
ResultsFrom 1428 eligible close contacts of infectious cases of TB, 303 (21%) met the exclusion criteria and were rejected from the analysis (Figure 1). From the remaining 1125 individuals, 578 (51%) had been screened in 2015 using the TST/IGRA strategy and 574 (49%) were screened in 2016 using the IGRA-only strategy. In order to assure that we were comparing the costs of screening contacts of the same number of TB cases, we included all 2016 TB cases and the same number of cases diagnosed in 2015 starting from the end of the year (79 contacts screened with TST/IGRA in January/February 2015 were excluded).
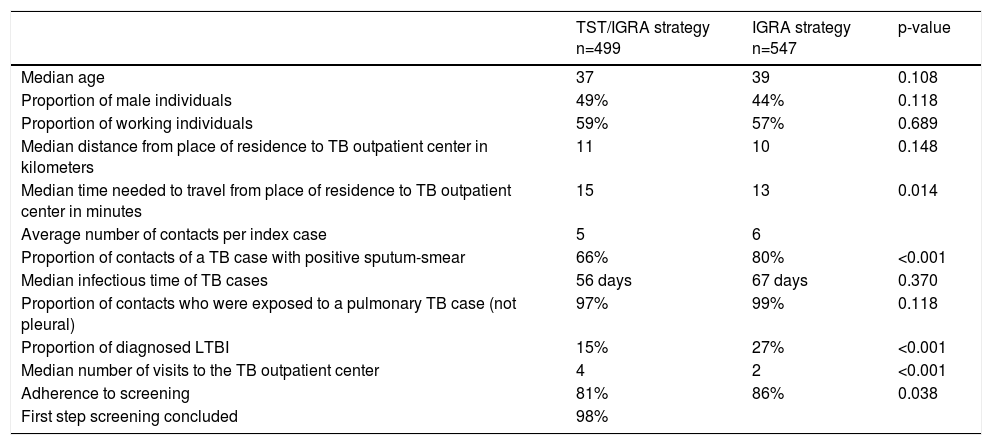
Baseline Comparison of GroupsThe median age of screened individuals was 38 years-old (interquartile range [IQR]: 25.0-51.0), 54% were women, and 58% (n = 641) were employed. Their places of residencies were a median of 11 km (IQR 6.5-15.5) and 14 min (IQR 8.5-19.4) away from the visited TB outpatient center. The two groups had no significant differences in terms of age, sex, employment status, and distance to the visited TB center (Table 1). However, the IGRA-only group had a significantly higher proportion of individuals who were exposed to highly infectious TB patients (positive sputum smears) (Table 1). The proportion of contacts diagnosed with LTBI and the adherence to screening were also greater in the IGRA-only group.
Characteristics of screened contacts by strategy used.
LTBI: latent tuberculosis infection.
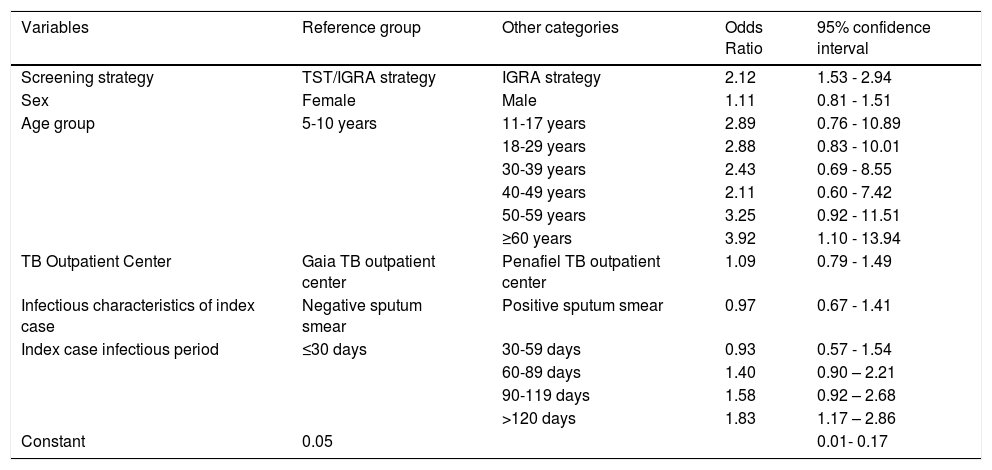
After adjusting for sex, age, TB outpatient center, index case sputum smear results, and period of infectiousness, the IGRA-only group had an increased risk for diagnosis of LTBI (aOR = 2.12, 95% CI: 1.48–2.93) (Table 2).
Logistic regression model for diagnosis of LTBI (final model).
LTBI: latent tuberculosis infection.
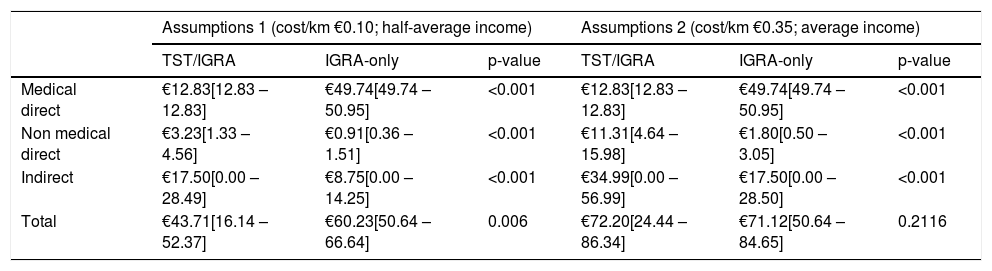
Total average costs were €42.71 per screened individual in the TST/IGRA group and €55.21 in the IGRA-only group; the corresponding median values were €43.71 and €60.23, respectively (Table 3). Medical direct costs were higher in the IGRA group, but non-medical direct costs and indirect costs were higher in the TST/IGRA group (Table 3). The cost per LTBI diagnosis was €280.42 in the TST/IGRA group (76 per 499 screened individuals) and €205.44 in the IGRA group (147 per 547 screened individuals). The estimated number of potentially averted cases of TB was 5 in the TST/IGRA group and 8 in the IGRA-only group. Thus, the cost per potentially averted TB case was €4412.48 in the TST/IGRA group (€21,312.29/4.83) and €3719.20 in the IGRA group (€30,199.87/8.12).
Screening costs by screening strategy (median [IQR], in EUR)
LTBI: latent tuberculosis infection. IQR: interquartile range.
After changing the previous assumptions regarding travel (cost/km of €0.35 instead of €0.10, and average daily income instead of average half-daily income), total costs were €64.48 per screened individual in the TST/IGRA group and €65.63 per individual in the IGRA-only group (median €72.20 and €71.12, respectively) (Table 3). The cost per LTBI diagnosis was €423.36 in the TST/IGRA group (76 in 499 individuals) and €244.22 in the IGRA group (147 in 547 individuals). The cost per potentially averted TB case was €6661.60 in the TST/IGRA group (€32,175.52/4.83) and €4421.13 in the IGRA group (€35,899.61/8.12). Medical direct costs were greater in the IGRA group, but non-medical direct costs and indirect costs were greater in the TST/IGRA group.
Incremental cost-effectiveness ratio (ICER)The calculated ICER was €106 per LTBI diagnosis, representing increased effectiveness with a slightly increased cost of IGRA-only screening strategy.
DiscussionOur comparison of two groups of close contacts of TB cases who followed different LTBI screening strategies showed that, when compared to the TST/IGRA group, the IGRA-only group had increased odds of having LTBI diagnosed (aOR = 2.12, 95% CI = 1.53–2.94) . Adherence to screening was also higher in the IGRA-only group, probably because this strategy requires fewer visits to the TB outpatient centers. From a societal perspective, the IGRA-only strategy appears to be more cost-effective than TST/IGRA strategy, because it has a lower cost per diagnosed LTBI case (€205.44 vs. €280.42) and a lower cost per potentially averted case of TB (€3,719.20 vs. €4,412.48).
The odds ratio for LTBI diagnosis was greater in the IGRA-only group than in the TST/IGRA group in Penafiel (high TB-incidence) than in Vila Nova de Gaia (medium TB-incidence). There is evidence that the TST and IGRA have similar sensitivity3,4 but the increased specificity of two-step strategies comes with a lower sensitivity. Previous studies showed that increasing age and immunosuppression are associated with false negative results, especially with TST.25 Other preconditions, like inflammatory diseases, might be associated with IGRA false negative results.26 Nevertheless, we used data from healthy individuals, >5 years old, without HIV infection, diabetes or pharmacological immunosuppression (table 1). We expect very few false positive results with the IGRA-only screening strategy, because of its high specificity, but no gold-standard test is available for confirmation.
Previous cost-effectiveness studies suggested that two-step screening was less effective averting active TB cases, but more cost-effective than IGRA-only screening.2 The present study also considered the effect of societal costs, and included not only medical costs but also non-medical direct and indirect costs. Our results suggests that the IGRA-only strategy is more cost-effective, mainly because of its higher effectiveness in diagnosing LTBI (and potentially averting TB cases) and decreased indirect costs (less productivity lost by individuals and society). As expected, the IGRA-only strategy represents increased costs for health services, because of the unit cost of the IGRA test itself and associated laboratory work. The incremental cost-effectiveness ratio of € 103 per LTBI diagnosis represents the amount of money spent for the outcome of interest. We considered effectiveness only for LTBI diagnosis, and not for treating LTBI (this was studied elsewhere11).
This may have led to an overestimation of the number of potentially averted cases of TB in both groups. A selection bias in the IGRA-only group may have occurred, because individuals in this group were exposed to more infectious TB cases (80% of index cases were sputum-smear positive in the IGRA-only group, but only 68% were sputum-smear positive in the TST/IGRA group). This might have occurred because the criteria used to identify eligible contacts were stricter for the more expensive screening strategy. However, including infectiousness in our regression model should have eliminated this bias.
ConclusionFrom a societal perspective, IGRA-only screening appears to be more cost-effective than TST/IGRA screening for LTBI, with a lower cost per LTBI diagnosis and a lower cost per potentially averted TB case. These results indicate an increased effectiveness of IGRA-only screening, at an only slightly increased cost.
There was no financial support to this study.