Typically, patients with progressive neuromuscular disorders (NMDs) develop acute respiratory failure (ARF), are intubated, and when failing spontaneous breathing trials (SBTs) undergo a tracheotomy and receive tracheostomy mechanical ventilation (TMV). However, increasing numbers of patients use nasal noninvasive ventilation (NIV), initially for sleep and this is extended to continuous dependence (CNVS). This can be used as a strategy to assist in successful extubation . We retrospectively reviewed 19 centers offering CNVS and mechanical insufflation-exsufflation (MI-E) as an alternative to TMV.
MethodsCenters with publications or presentations concerning CNVS outcomes data were pooled for amyotrophic lateral sclerosis (ALS), Duchenne muscular dystrophy (DMD), and spinal muscular atrophy type 1 (SMA1). Progression to CNVS dependence without hospitalization, duration of dependence, and extubations and decannulations to CNVS were recorded. Prolongation of life was defined by duration of CNVS dependence without ventilator free breathing ability (VFBA).
ResultsThere were 1623 part time (<23 h/day) NVS users with ALS, DMD, and SMA1 from 19 centers in 16 countries of whom 761 (47%) were CNVS dependent for 2218 patient-years. This included: 335 ALS patients for a mean 1.2 ± 1.0 (range to 8) years each; 385 DMD patients for 5.4 ± 1.6 (range to 29) years; and 41 SMA1 patients for 5.9 ± 1.8 (range to 20) years. Thirty-five DMD and ALS TMV users were decannulated to CNVS and MI-E. At data collection 494 (65%) patients were CNVS dependent but 110 (74 of whom with bulbar ALS), had undergone tracheotomies.
ConclusionsALS, DMD, and SMA1 patients can become CNVS dependent without requiring hospitalization but CNVS cannot be used indefinitely for many patients with advanced upper motor neuron diseases.
There have now been over 100 peer-reviewed articles, reviews and consensus papers on sleep and noninvasive ventilation (NIV). “NIV” has become synonymous with continuous positive airways pressure (CPAP) or bi-level positive airway pressure (BiPAP). All reported benefits but, there have not been any reports highlighting NIV for full ventilatory support. Nor, are there reports of the use of MIE with noninvasive ventilatory support (NVS) settings used in the 1950s as an alternative to TMV.1 Survival is usually reported to be prolonged on the basis of randomizing sleep NIV with untreated cohorts. However, patients continue to weaken and develop ARF during respiratory tract infections irrespective of NIV use, when intubated, these patients often fail to wean and they undergo tracheotomy. However, in patients who lose all ventilator free breathing ability (VFBA), they typically have less than 100 ml of vital capacity (VC) and are apneic upon ventilator disconnection. Some centers have demonstrated that continuous noninvasive ventilatory support (CNVS) along with mechanical insufflation-exsufflation (MI-E) can be used indefinitely instead of tracheostomy mechanical ventilation (TMV) and ventilator “unweanable” patients can be extubated and decannulated to CNVS and MI-E.
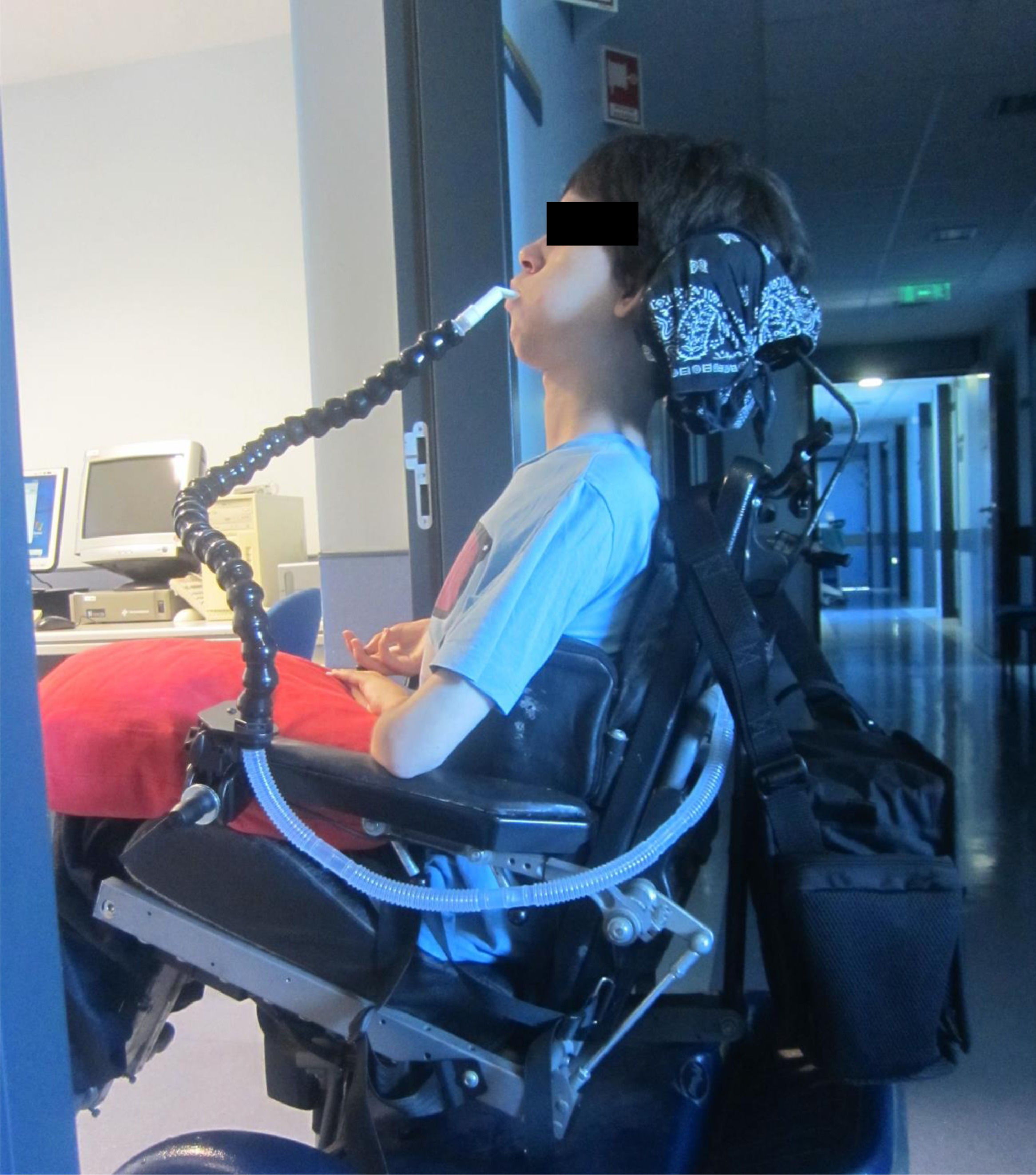
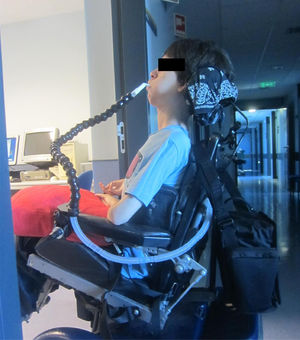
Although in 1987 we first published CNVS via a nasal interface for a multiple sclerosis patient with no VFBA and 100 ml of VC,2 with the release of a bi-level pressure cycled mode (BiPAP™, Respironics Inc., Murrysville, Pa) devices in 1990, NMD patients are being placed on BiPAP at less than NVS and full respiratory muscle rest settings. With advancing disease, some have had their sleep bi-level mode settings increased and were commenced on daytime mouthpiece NVS with a volume-cycled mode, however, once intubated for any reason they received a tracheotomy rather than continue CNVS.3-7 Typically, most clinicians still do not offer diurnal mouthpiece (Fig. 1) and nasal NVS, when mask NIV use exceeds 15–20 h and tracheostomy is therefore recommended.
Indeed, even in 2015 diurnal mouthpiece NVS was described as a “novelty”8 despite the fact that CNVS via mouthpiece was first described in 1953 and 257 such cases were reported in 1993.4,9,10 In 1953, Dr. John Affeldt wrote that patients can have a simple mouth piece “…hang in the mouth, we even had one patient who has no breathing ability who has fallen asleep and been adequately ventilated by this procedure."9
In 1956 Hodes noted, “…tracheotomy may be a great disadvantage. It is very difficult to get rid of a tracheotomy tube when the VC is only 500 or 600 cc and there is no power of coughing, whereas, as we all know, a patient who has been treated in a respirator (body ventilator) from the first can survive and get out of all mechanical devices with a VC of that figure."11 Thus, it was understood that tracheostomies could increase ventilator dependence because of inspiratory muscle deconditioning, tube induced secretions, hyperventilation by bypassing upper airway afferents, and possibly other factors.12 As a result of TMV complications, a switch back to noninvasive approaches began in the 1980s.13,14 However, CNVS and MI-E can only be a definitive alternative to TMV if patients can be extubated back to them during intercurrent intubations for ARF which several centers have reported doing.15,16
Airway secretion congestion, especially during intercurrent respiratory tract infections, causes 90% of episodes of ARF and intubations for Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) type 1 patients,6,17,18 and results in tracheotomies19–21 when CNVS and MI-E are not used17,22–24 to facilitate extubation.15,16 Unlike invasive suctioning, MI-E does not favor right over left airways.25–27 It can also be used via invasive airway tubes and has been reported to be critical for the successful extubation/decanulation of “unweanable” patients13,16 as well as decreasing hospitalization rates.17,22,28
Thus, while the benefits of Bi-level NIV have been reported to be many but brief,29–31 the purpose of this work is to demonstrate that CNVS can be used indefinitely as an alternative to TMV to prolong survival for years for patients with advanced NMD and patients can become dependent on CNVS with no VFBA without hospitalization.
MethodsThe participating centers had a multidisciplinary team that included medical doctors and/or respiratory (physio)therapists with more than 10 years of experience in CNVS and MI-E in neuromuscular disorders with scientific presentations and peer-reviewed publications in these areas of research. Some centers also reported extubating ventilator unweanable patients to CNVS and MI-E. The participating clinicians obtained consent for the chart reviews from their Institutional Review Boards (IRBs) or ethics committees, however, three of the centers did not have them.
Nocturnal nasal NVS or Bi-level mode at NVS settings of greater than 15 cm H2O pressure support were introduced to patients with symptomatic hypoventilation. When symptoms were unclear, SpO2/end-tidal, transcutaneous carbon dioxide (CO2) monitoring, or polysomnography was performed depending on the particular center. Typically, settings were prescribed over a volume range of 700–1500 ml or assist-control or pressure support pressures of 15–25 cm H2O and therapists worked with patients to choose their desired settings. Physiologic back-up rates for age were prescribed. No expiratory PAP or positive end-expiratory pressure (PEEP) was used in centers where portable ventilator circuits had an active valve circuit, otherwise bi-level devices were used at NVS settings. According to clinical indication, during nocturnal NVS, all patients used active external humidification devices.32
The extent of need for NVS was divided into Grades of 0–4. Patients with grades of 1–4 can become dependent on CNVS and MI-E temporarily or permanently when acutely ill. Grade 4 CNVS dependent patients become dyspneic with O2 desaturation within seconds to minutes of disconnection and cannot survive without it (Fig. 1).
While European and North American centers typically used homecare life support ventilators (pressure and volume modes with internal batteries), these were often too expensive for South and Central American centers. These centers used homecare bi-level devices, with IPAP's typically to 20–25 cm H20. including, at times, for daytime mouthpiece NVS. Bi-level devices were used at NVS settings with inspiratory pressures over 15 cm H2O and minimum EPAP's. In all centers, patients performed LVR using manual resuscitators and volume cycling during the day. Nasal interfaces were used for sleep and often for daytime NVS. South American centers generally used MPV via a BiPAP device via angled mouthpieces with an HME in the circuit. More recently, the homecare life support ventilators have become more available but, are used with less expensive, disposable passive circuits for diurnal mouthpiece NVS with volumes of 700–1500 mls. Acutely ill intubated patients of the centers underwent tracheotomies elsewhere.
Likewise, the standard MI-E devices are generally unavailable in South America so inexpensive, locally produced MI-E devices and manually assisted coughing are used. As a result, MI-E-exsufflation flows (MI-E-EF) cannot usually be measured. A manual resuscitator and abdominal thrusts are used for manually assisted coughing. Patients are typically hospitalized to initiate NIV but some were also set up at regular out-patient visits, or in their homes depending on the center.
The data for DMD, ALS, and SMA1 were collated to determine duration of part-time (Grades 1–3) and for Grade 4 or CNVS dependence. Progression to CNVS without hospitalization, and numbers of extubations and decannulations of patients who could not pass SBTs before or after extubation or decanulation were also collate. Prolongation of life was quantitated by duration of dependence on CNVS such that discontinuing it resulted in acute respiratory distress, blood gas deterioration, and certain death without quickly returning to it. Most had VCs of 0 to 250 ml but only a few centers monitored spirometry.
Descriptive data are reported as mean ± standard deviation (SD) with analysis performed by SPSS software (Release 24.0 SPSS, Chicago, I1, USA).
ResultsOf the 23 centers contacted, 18 took part from 16 countries. Clinicians from five centers declined to participate either in order to publish independently (3 cases) or because of inadequate resources to gather data. Thirty-two clinicians from the 19 centers had 1623 NVS users.
The patients with Grades 1–3 dependence, their ages at onset and duration of part-time use, the number advanced to CNVS dependence and its duration, current age, the extubations and tracheostomy tube decannulations to CNVS, and the deaths while using CNVS or after undergoing tracheotomy are noted in Tables 1–3.
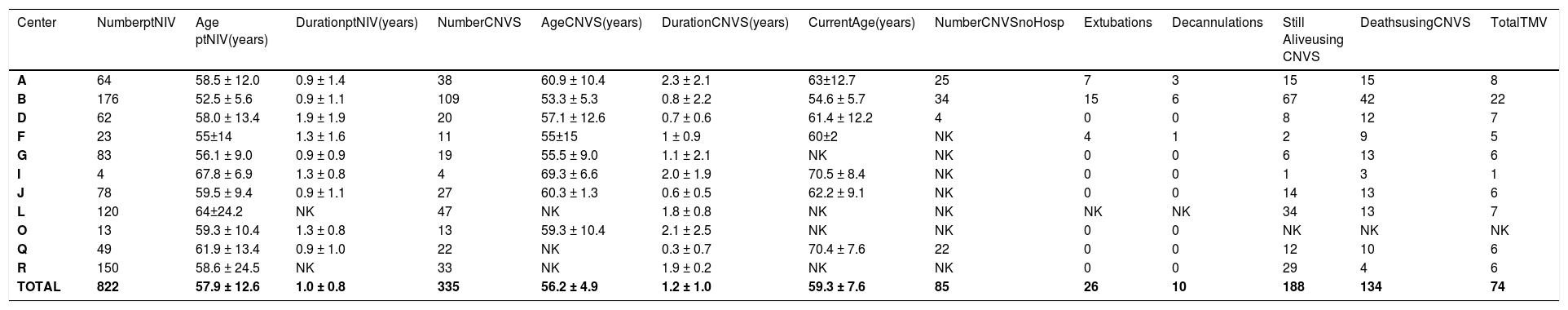
Data on Amyotrophic lateral sclerosis.
Legend – Number ptNIV – number of patients using part time (<23 hr/day) noninvasive ventilation (NIV); Age ptNIV- age when beginning part-time NIV; Duration ptNIV- time of use of part time noninvasive ventilation (NIV); Number CNVS - number of patients progressing to full time dependence on continuous noninvasive ventilator support; Duration CNVS- duration of dependence on full time CNVS; Current Age - age currently or at time of death; Still Alive- number of CNVS users still alive; Extubations/Decanulations - Number of extubations/decanulations of “unweanable” patients to full-setting CNVS; Deaths – deaths when using CNVS or after undergoing tracheotomy for ventilatory support; TMV – number of CNVS patients undergoing tracheotomy; NK – not known.
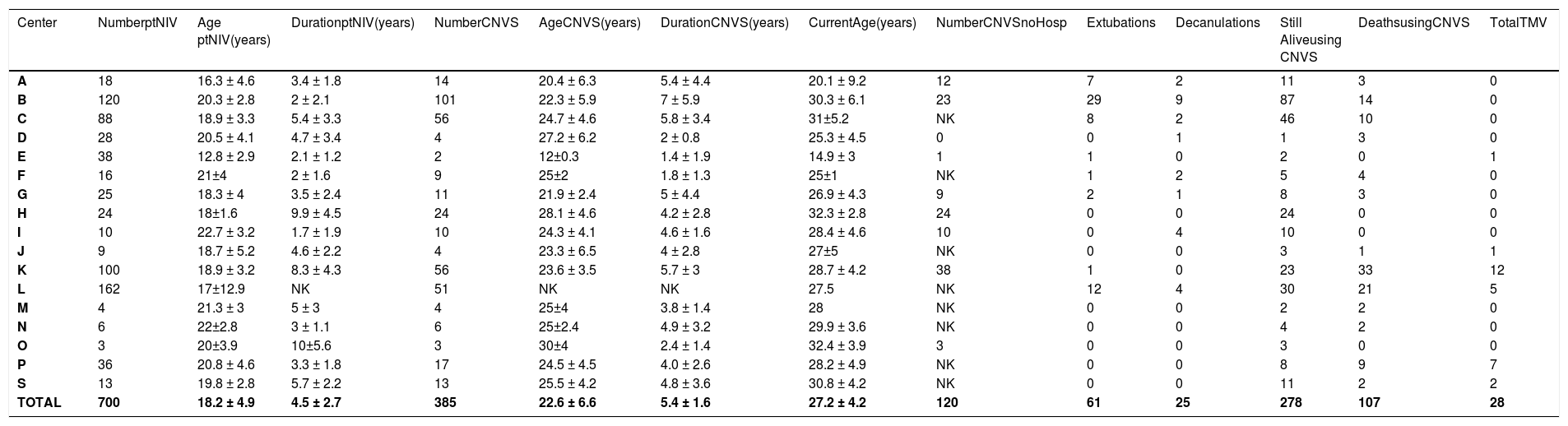
Data on Duchenne muscular dystrophy.
Legend – Number ptNIV – number of patients using part time (<23 hr/day) noninvasive ventilation (NIV); Age ptNIV- age when beginning part-time NIV; Duration ptNIV- time of use of part time noninvasive ventilation (NIV); Number CNVS - number of patients progressing to full time dependence on continuous noninvasive ventilator support; Duration CNVS- duration of dependence on full time CNVS; Current Age - age currently or at time of death; Still Alive- number of CNVS users still alive; Extubations/Decanulations - Number of extubations/decanulations of “unweanable” patients to full-setting CNVS; Deaths – deaths when using CNVS or after undergoing tracheotomy for ventilatory support; TMV – number of CNVS patients undergoing tracheotomy; NK – not knowned; S – Data from Metro-Health Medical center, Case Western Reserve University, USA gathered by Dr. David Birnkrant with great appreciation.
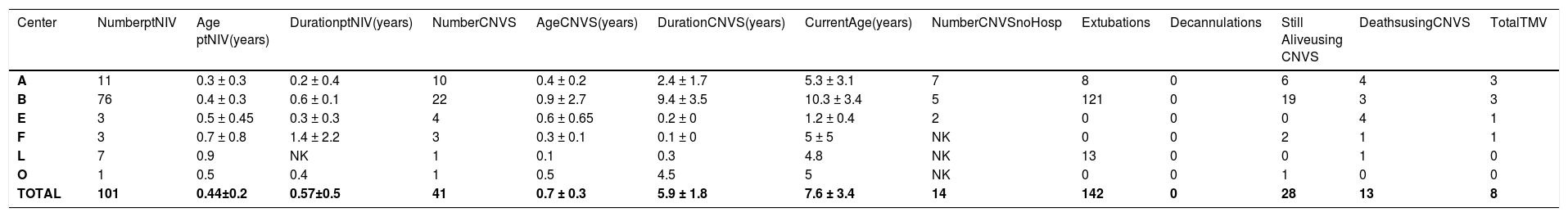
Data on Spinal muscular atrophy type 1.
Legend – Number ptNIV – number of patients using part time (<23 hr/day) noninvasive ventilation (NIV); Age ptNIV- age when beginning part-time NIV; Duration ptNIV- time of use of part time noninvasive ventilation (NIV); Number CNVS - number of patients progressing to full time dependence on continuous noninvasive ventilator support; Duration CNVS- duration of dependence on full time CNVS; Current Age - age currently or at time of death; Still Alive- number of CNVS users still alive; Extubations/Decanulations - Number of extubations/decanulations of “unweanable” patients to full-setting CNVS; Deaths – deaths when using CNVS or after undergoing tracheotomy for ventilatory support; TMV – number of CNVS patients undergoing tracheotomy; NK – not known.
Centers:.
A – Department of Pulmonology, University Hospital of S. João, Faculty of Medicine, University of Porto, Portugal.
B - Department of Physical Medicine and Rehabilitation, Rutgers University - New Jersey Medical School, U.S.A.
C - National Organization Yakumo Hospital, Hokkaido, Japan.
D - Instituto de Investigaciones Médicas Alfredo Lanari, Facultad de Medicina, Universidad de Buenos Aires, Argentina.
E - Domiciliary Noninvasive ventilation Program, Health Chilean Ministry, Departments of Pediatrics and Medicine, Universidad de Chile, Santiago de Chile.
F - VentLar Home Care Program, Fundação Hospitalar Estado Minas Gerais (FHEMIG), Belo Horizonte, Brasil.
G - Respiratory Rehabilitation unit, Ottawa University Hospital, Canada.
H - Neuromuscular Unit, Nigrisoli Hospital, Bologna, Italy.
I - Department of Rehabilitation Medicine, Gangnam Severance Hospital, Seoul, South Korea.
J - Department of Respiratory Medicine, Hospital Clínico Valência, Spain.
K - Center for Home Mechanical Ventilation, Indekaal Rehabilitation Hospital, Brussels, Belgium.
L - Sleep and Ventilation Unit, Royal Brompton Hospital, London, UK.
M - Asklepios Klinik Hamburg-Harburg, Germany.
N - Centre Suisse des Paraplégiques, Nottwil, Switzerland.
O - Tung Wah Hospital, Hong Kong, China.
P - Pulmonary Division, Sleep Disorders and Neuromuscular Center, University Hospital of Zurich, Switzerland.
Q – Medical Diagnostic Inc., St. Petersburg, Florida, USA.
R - Tel Aviv Sourasky Medical Center, Tel Aviv University medical school, Israel.
Of the 1623 part-time nasal NVS users, 761 progressed to CNVS dependence with no VFBA for 2218 patient-years of prolonged survival, 1148 patient-years for DMD, 577 for ALS, and 193 for SMA 1, without tracheostomies. At least 219 patients reported by 6 of the 19 centers progressed to CNVS without being hospitalized or developing ARF. This included 120 DMD patients from 6 centers, 85 ALS patients from 4 centers, and 14 SMA 1 patients from 3 centers. Since NVS was only begun for symptomatic hypoventilation, patients were told to use it only if benefits outweighed inconvenience. Since a variety of nasal, oro-nasal, and mouthpiece interfaces was offered, intolerance was limited to advanced bulbar ALS patients whose principal respiratory symptoms were due to stridor from upper airway collapse and airway secretion congestion rather than from hypoventilation. No DMD or SMA1 patients failed to tolerate NVS, and subsequently CNVS, and there were no skin or other complications that prevented use.
Only 8 of the 19 centers extubated/decannulated patients who failed all weaning parameters to CNVS and MI-E.16 Of 255 patients who were unweanable before and immediately after extubation, 252 were successfully extubated so only 3 ALS patients underwent tracheotomy due to multiple extubation failures in the 8 centers.
At the time of data collection 110 CNVS users had undergone tracheotomy, 108 of whom as a result of an episode of ARF not managed by the 8 centers that used the extubation protocol.16 Thus, all of the DMD and most of the SMA1 patients who ultimately underwent tracheostomy did so at non-participating centers. Outcomes as a function of diagnosis are listed in Tables 1–3.
DiscussionOur results demonstrate that CNVS can prolong life for years for patients with little to no VFBA for patients without upper motor neuron signs and without resorting to tracheostomy. CNVS can even be used indefinitely for patients with little or no bulbar-innervated muscle function or vital capacity (VC) as for the SMA type 1 patients, none of whom had upper motor neuron signs. Indeed, while DMD and SMA1 patients used CNVS to prolong survival for over 5 years, significantly longer than for ALS (p < 0.001), and many with SMA1 did so despite absence of all bulbar-innervated muscle function and VC, most ALS patients could not become CNVS dependent despite retaining some bulbar-innervated muscle function. Unlike the DMD and SMA1 patients, the ALS patients had stridor.
CNVS and MI-E can only replace inspiratory and expiratory muscle function. With severe bulbar involvement, the SMA type 1 patients have to be positioned to drool rather than aspirate and, indeed, since the initial data collection, these centers now have more than 15 CNVS dependent SMA1 patients over 20 years of age without tracheostomy tubes, despite CNVS dependence from as young as 4 months of age and no measurable VC. On the other hand, when ALS patients develop stridor, decreased MI-E-EF, and a decrease in baseline O2 saturation, tracheostomy becomes necessary for survival.33–36 Thus, although the majority of DMD patients’ and SMA1 patients’ lives could be prolonged by CNVS, this was not the case for the majority of ALS patients.23,30,37–39 This is the first multicenter report on prolonging life by CNVS that includes patients with little to no VFBA, indeed, some with no measurable VC.23,31,37–40 Besides DMD, ALS, and SMA1 CNVS users, all of the centers had CNVS users with other NMD diagnoses as well.42–46
Thus far, life has been extended to over age 50 for DMD by CNVS,7 and by a mean of 5.4 years for 385 DMD patients in this study,6,41,42 to age 27 for SMA1 by dependence on CNVS from as young as 4 months of age,43,44 and by a mean of over 1 year for 25 to 42%, and to a maximum of 8 years, for ALS patients.45 Such outcomes suggest that commonly reported “NIV failure”46–48 can result from inadequate NIV settings, failure to use MI-E at adequate settings, and failure to use diurnal NVS5 and air stacking.49 This explains the “preference” for tracheostomy for patients not offered CNVS and MIE.50,51
Whereas special expertise is required for noninvasive management, since 19% of 157 successfully extubated unweanable patients in one study had critical care neuromyopathies and not NMD, the expertise should not be limited to isolated centers. There should also be no “inability of local medical infrastructure to support NIV”52 except in countries where ventilators are not funded at all.
The impact of disease and ventilatory dependence on patients' health, daily life and well-being should also be measured directly from the patients themselves, by means of validated health status questionnaires. It is important to apply short, valid and easy to use tools to monitor NVS in clinical practice to promote a more efficient organization of home mechanical ventilation services.53
Virtual reality may also be a promising technology for implementing personalized, motivating and controlled strategies for home mechanical ventilation in severe respiratory patients. These include, for example, mobile applications for the self-management and tele-monitoring systems, allowing personalized treatments and monitoring.54
In conclusion, ventilator dependent patients with no VFBA cannot survive without continuous ventilatory support. This is now being provided noninvasively by using CNVS supplemented by MI-E in some centers. Patients unable to pass SBTs can also be successfully extubated to CNVS and MI-E. MI-E needs to be used in critical care; staff needs to be instructed in CNVS and MI-E; and respiratory therapists need to be specifically trained and given the time needed to train patients. Since TMV is associated with poorer survival in DMD,6,55 and poorer quality of life40,56 than is CNVS, these outcomes beg for wider application.
Piepers et al. pointed out that “Analysis of subgroups of patients with disease (ALS) that benefit more from NIV, and the timing of introduction of this treatment in patients with severe bulbar impairment, should be the subject of future study.57 ”We can now suggest that most ventilator dependent ALS patients cannot utilize CNVS because of the upper motor neuron signs that cause stridor and that this does not occur in DMD or SMA.
Key practical points of major strength of this study include:
- 1)
Few if any patients with myopathic conditions or lower motor neuron disease who have access to CNVS and MIE should ever require tracheotomies.
- 2)
Upper motor neuron bulbar ALS patients require tracheotomies when they develop stridor with low MIE-EF and O2 saturation baseline below 95% with CNVS.
- 3)
Other than for ALS patients, ventilator unweanable patients with NMD can be extubated to CNVS and MIE with an over 99% success rate and thereby avoid tracheotomies.
- 4)
Only 25–45% of ALS patient can become CNVS dependent, but they, and the DMD and SMA 1 patients can do so without being hospitalized, developing ARF, or being intubated.
The authors would like to thank Michelle Chatwin Ph.D for her invaluable help in reviewing this manuscript.