Asthma is a common, chronic and heterogeneous disease; it affects people of all ages and there has been a recent increase in its prevalence and severity. It may be mild, barely noticed by the patient, or it may range all the way to very severe disease, causing constant symptoms that greatly affect the quality life of the patient. The long-term goals of asthma management are to achieve good symptom control and minimize future risk of exacerbation, fixed air flow limitation and treatment of side effects.1 The patient own goals regarding their asthma and its treatment should also be identified. During the last decade several new drugs addressing asthma have been developed and approved.
The immune system is a complex interactive network with the capacity of protecting the host from a number of pathogens while maintaining a state of tolerance to self and innocuous exogenous antigens. Allergy is one of the immune tolerance-related diseases that arises as a direct consequence of a dysregulated immune response.
Currently, allergen-specific immunotherapy (allergen-SIT) remains the single curative approach to allergic diseases with the potential to modify its course.2 It is proven to be the only therapy that alters the natural history of allergic disease, prevents progression, prevents the development of new sensitizations and may even prevent the development of asthma in patients with allergic rhinitis.3 It consists of administering gradually increasing doses of a specific allergenic extract to an allergic subject (with specific documented IgE), at an effective dose, in order to decrease the symptoms associated with subsequent exposure to the causative allergen.3 Multiple mechanisms are involved in the suppression and/or control of allergic inflammation.4 The aim of allergen-SIT is to induce the peripheral T cell tolerance, modulate the thresholds for mast cell and basophil activation and decrease IgE-mediated histamine release.4 Peripheral T cell tolerance is characterized by the generation of allergen-specific T-regulatory cells (Treg) that are able to produce anti-inflammatory cytokines such as IL-10 and TGF-b.5 Treg cells not only diminish Th2 immune responses, but also target other cell types such as mast cells, basophils, eosinophils, allergen-specific-IgE and are capable of inducing IgG4 and IgA production.6
A review of Abramson et al., which included eighty-eight trials, showed a significant reduction in asthma symptoms, reduction in use of medications and improvement in bronchial hyper-reactivity following immunotherapy.7 According to the Global Initiative for Asthma (GINA) 2017, for patients with allergic rhinitis and sensitization to house dust mite, with exacerbations despite low-high dose of ICS, we must consider adding sublingual allergen immunotherapy (SLIT), provided Fev1 is >70% predicted.1 Thus, in the latest GINA recommendations, allergen-SIT have been included for patients with exacerbation despite taking step 3 or step 4 therapy, as an add-on therapy.1
Here, we do a prospective analysis of patients with asthma submitted to allergen-SIT followed in allergology appointment during 5 years (2013–2017). Statistical analysis was performed using the IBM SPSS® statistical program, version 25. The variables were described with mean, median, standard deviation, maximum and minimum. All the tests were considered significant when p-value did not exceed 0.05.
We included 35 patients with mean age 28.1±11.4 years. The majority (94.3%) had associated allergic rhinitis. Regarding therapy 29% of the patients performed allergen-SIT for dermatophagoides pteronyssinus (Dpt), 23% Dpt/dermatophagoides farinae, 14% Dpt/Lepidoglyphus destructor and 31% for Gramineae mixture. The majority had allergenic polysensitization (57%).
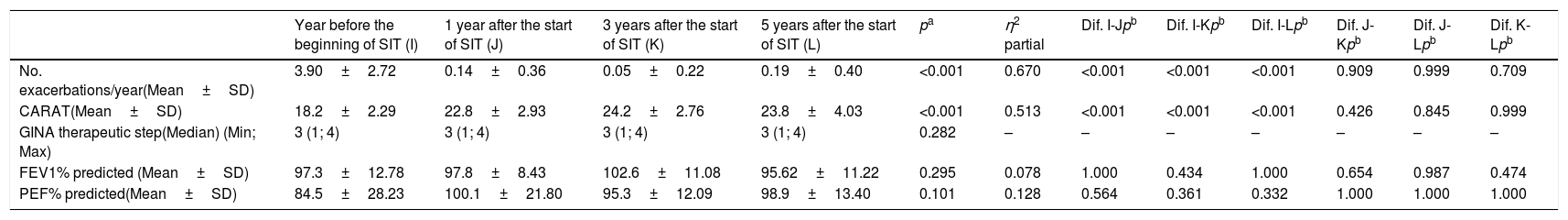
In the year before the start of allergen-SIT, patients presented an average of 3.9±2.7exacerbations/year and mean CARAT of 18±2.3. One year after the onset of allergen-SIT, they presented a mean of 0.14±0.36exacerbations/year and mean CARAT of 22.8±2.9. At 3 years, they presented a mean of 0.05±0.22exacerbations/year and mean CARAT of 24.2±2.7, whereas at 5 years, they presented a mean of 0.19±0.40exacerbations/year and mean CARAT of 23.8±4. During this period they were performing therapy on step 3 of the GINA. There were statistically significant differences in the number of exacerbations/year and the CARAT questionnaire mean at 1, 3 and 5 years (p<0.001). There were no statistically significant differences in bronchodilator and anti-inflammatory therapy during the same period (p=0.282), Table 1. Regarding safety, most local adverse reactions were mild and there were neither life-threatening systemic reactions nor fatal events.
| Year before the beginning of SIT (I) | 1 year after the start of SIT (J) | 3 years after the start of SIT (K) | 5 years after the start of SIT (L) | pa | η2 partial | Dif. I-Jpb | Dif. I-Kpb | Dif. I-Lpb | Dif. J-Kpb | Dif. J-Lpb | Dif. K-Lpb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. exacerbations/year(Mean±SD) | 3.90±2.72 | 0.14±0.36 | 0.05±0.22 | 0.19±0.40 | <0.001 | 0.670 | <0.001 | <0.001 | <0.001 | 0.909 | 0.999 | 0.709 |
| CARAT(Mean±SD) | 18.2±2.29 | 22.8±2.93 | 24.2±2.76 | 23.8±4.03 | <0.001 | 0.513 | <0.001 | <0.001 | <0.001 | 0.426 | 0.845 | 0.999 |
| GINA therapeutic step(Median) (Min; Max) | 3 (1; 4) | 3 (1; 4) | 3 (1; 4) | 3 (1; 4) | 0.282 | – | – | – | – | – | – | – |
| FEV1% predicted (Mean±SD) | 97.3±12.78 | 97.8±8.43 | 102.6±11.08 | 95.62±11.22 | 0.295 | 0.078 | 1.000 | 0.434 | 1.000 | 0.654 | 0.987 | 0.474 |
| PEF% predicted(Mean±SD) | 84.5±28.23 | 100.1±21.80 | 95.3±12.09 | 98.9±13.40 | 0.101 | 0.128 | 0.564 | 0.361 | 0.332 | 1.000 | 1.000 | 1.000 |
In conclusion, after one year of allergen-SIT, we verified a significant symptomatic improvement in the clinical control of asthma, as evidenced by the statistically significant difference in the number of exacerbations/year and in the CARAT questionnaire. This control remains stable after 3 and 5 years of therapy, without significant differences in bronchodilator and anti-inflammatory therapy. Therefore, the current evidence provides support for the efficacy and safety of allergen-SIT in the treatment of respiratory allergy, namely in clinical control of allergic asthma and rhinitis, representing a safe alternative or an add-on therapy to conventional inhaler therapy. However, it is essential to select patients to undergo this treatment and which extracts to be used to optimize the cost/benefit.
Conflicts of interestThe authors have no conflicts of interest to declare.