Treatment of interstitial lung disease (ILD) in primary Sjogren's Syndrome (pSS) is a clinical need which still needs to be defined.1 Our aim in the following paragraphs is to report the case of a patient with pSS and acute onset ILD successfully treated with Rituximab (RTX).
On May 2020, a 49 years-old former smoker Caucasian male was hospitalized for recent-onset of dyspnea. On assessment, the patient did not present pyrexia and the only past medical history was arterial hypertension.
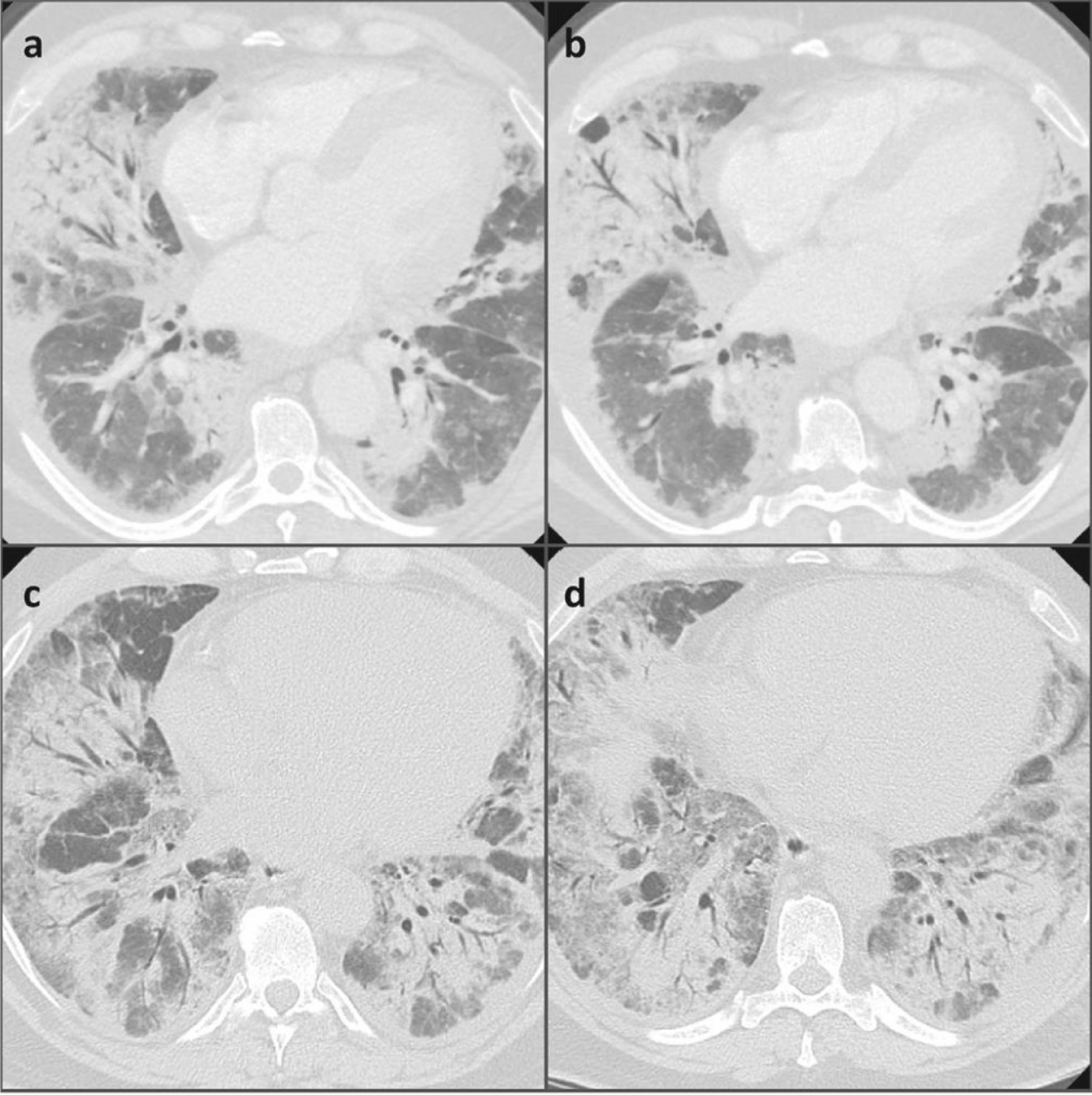
A negative nasopharyngeal-throat swab excluded the SARS-CoV2 infection. The erythro-sedimentation rate was high (64 mm), whereas the C-reactive protein, the procalcitonin, the total leukocyte count and the renal and hepatic function were normal. The first chest high resolution computed tomography (HRCT) showed diffuse and bilateral ground glass opacities with thickened interlobular septa all over the lungs, which were suggestive of an infectious disease. For this reason, we started an empiric antibiotic therapy with amoxicillin/clavulanic acid and subsequently with levofloxacin, in association with oxygen supplementation (Fig. 1). No positivity was revealed on gargled samples for bacteria, viruses or other germs.
The patient presented a rapid worsening of respiratory failure needing support with high flow nasal oxygen (HFNO). Pneumocystis jirovecii was isolated in broncho-alveolar lavage (BAL) with a low replication load, so we increased the antibiotic therapy with trimethoprim/sulfamethoxazole. Because of disease severity vancomycin was also added to cover nosocomial pathogens. The BAL cellular analysis resulted nonspecific: it revealed mostly foamy macrophages.
Nevertheless, the patient's clinical and radiological features continued to deteriorate. The patient was transferred to the Intensive Care Unit where non-invasive mechanical ventilation was added to HFNO due to clinical deterioration with associated respiratory failure and also radiological worsening with finding of increased ground glass opacities in the chest HRCT scans.
Although a partial resolution of the diffuse basal thickening could be noticed on the chest HRCT, respiratory failure persisted. Therefore, the patient was transferred to our Respiratory Disease Unit, where other diagnostic investigations were performed.
Autoimmunity tests resulted in a marked positivity of anti-SSA/Ro-52 kDA, whereas rheumatoid factor, anti-citrullinated peptides antibodies were negative, and C3 and C4 fractions of complement were normal. Transbronchial biopsies performed earlier found foci of organizing pneumonia (OP).
A minor salivary glands biopsy was performed for the differential diagnosis of a mild sicca syndrome, documenting a salivary focus score >1.
In light of these results and after a multi-disciplinary discussion including pulmonologists and rheumatologists, the patient was diagnosed with pSS and prescribed a treatment with intravenous RTX (375 mg/m2 once a week for four weeks) and prednisolone (1 mg/kg/daily).
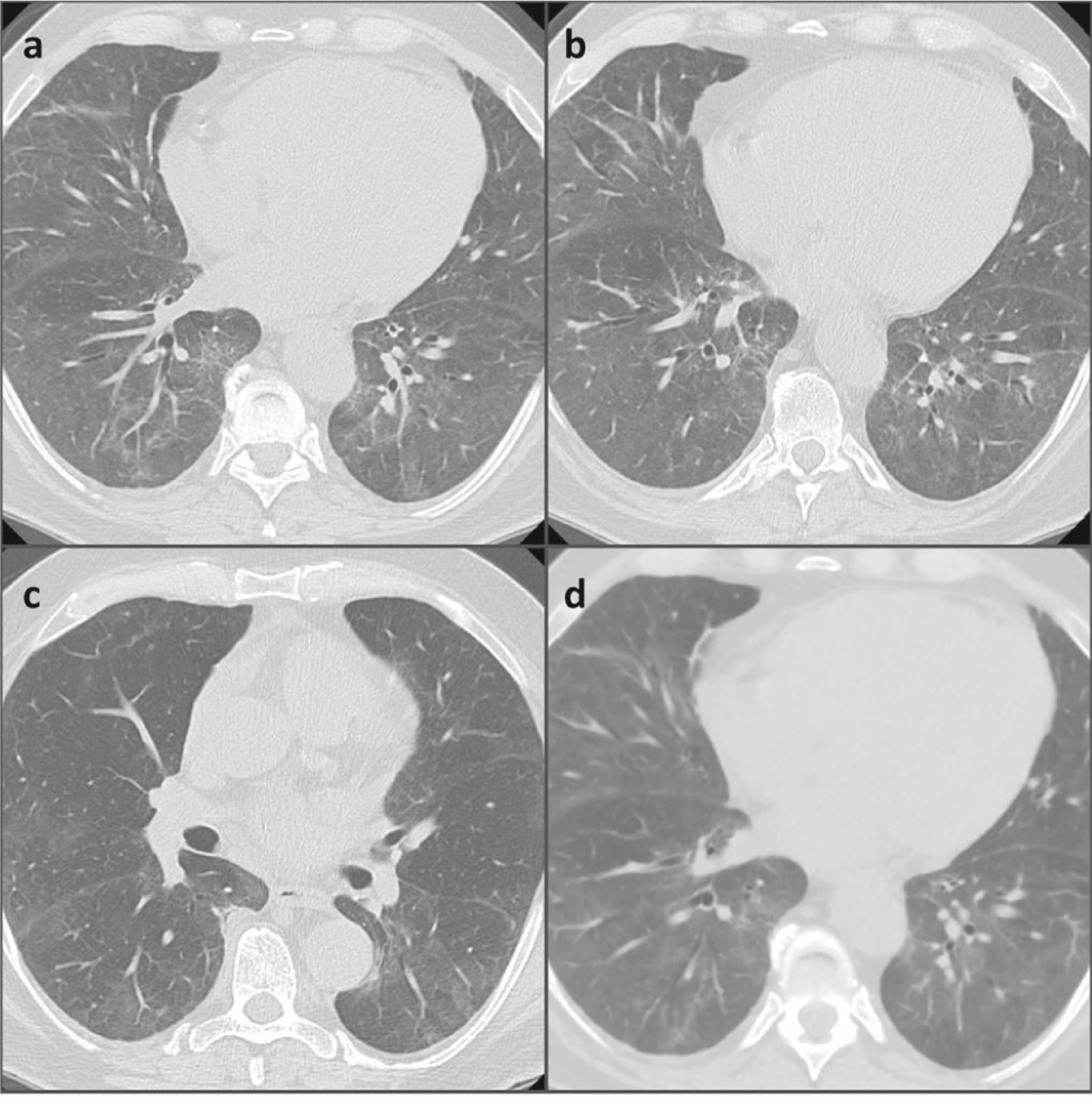
After a month, a new chest HRCT showed a significant improvement in the lung parenchymal involvement and the respiratory failure was resolved (Fig. 2). Finally, mycophenolate mofetil (MMF) was added to the treatment as steroid-sparing and maintenance drug.
PSS is a chronic inflammatory autoimmune disease characterized by lymphocytic infiltration of exocrine glands, mainly salivary and lachrymal glands, leading to progressive loss of glandular secretory function, resulting in eye and mouth dryness (sicca syndrome).2
In Europe, the annual incidence of pSS is estimated between 3.9 and 5.3 individuals per 100.000 people. There is a female to male ratio of 9:1 and a peak incidence in the fourth and fifth decades of life.3
Many extra-glandular organs and systems can be involved in pSS, including the lungs, the kidneys, small vasculature and the central nervous system.2 The prevalence of pulmonary involvement in pSS has been reported to be 9–70%, depending on the detection method and classification used. PSS-ILD is the most frequent form of pulmonary involvement and it has been observed in 3–11% of patients with pSS. Moreover, pSS related ILD is associated with a reduced quality of life and it is responsible for premature mortality.3
Our patient showed an OP pattern, which is the second most frequent ILD histological pattern in pSS after nonspecific interstitial pneumonia. Other less common patterns are usual interstitial pneumonia and lymphocytic interstitial pneumonia. Diagnosis of ILD is usually made late in the disease's clinical history (with a reported prevalence of 47% after 15 years of pSS onset4); however, more recently, many authors described ILD as a possible early complication of pSS, sometimes preceding the onset of the sicca syndrome. In this context, Roca et al. reported that 10–51% of patients developed ILD years before the onset and diagnosis of pSS, while in about 20% of patients the diagnoses of pSS and ILD are concurrent.5
Wang et al. reported that aging, cigarette smoking and ANA positivity may be potential risk factors associated with lung involvement in pSS. Previous studies involving systemic sclerosis, anti-synthetase syndrome and mixed connective tissue disease showed that anti-SSA/Ro antibodies are associated with ILD.6 Burvy et al. reported that anti-Ro52 antibodies are an independent risk factor for ILD in pSS as well.7
In patients with acute onset-ILD, anti-Ro52 antibodies may represent the only sign suggesting an underlying pSS.4
Up to now, immunosuppressive treatment has been the main strategy in ILD associated to connective tissue diseases (CTD). Due to the well-known involvement of B cells in pSS pathogenesis, we decided to treat our patient with RTX, while MMF was proposed as maintenance therapy. The latest European League Against Rheumatism (EULAR) recommendations8 do not advise the use of one immunosuppressive treatment over another, and, due to the lack of evidence, management for pSS-ILD remains empiric and dependent on the medical team's experience.3 Nevertheless, previous studies have demonstrated that RTX is a safe and useful drug in both proposed regimens.9 In particular, a French study on 78 pSS patients,10 treated the systemic involvement with 1 g every other week for two infusions in 86% of patients, while administering four infusions, once a week, of 375 mg/m2 in 14% of cases. In the AutoImmune and RTX registry, six of the eight pSS patients with ILD treated with RTX reported an improvement of pulmonary involvement.10 In 2016, Roca et at. reported a good response to RTX in one pSS patient with steroid refractory ILD.5 Furthermore, Chen et al. retrospectively investigated the effects of RTX in 10 pSS-ILD patients, showing improvement in DLCO and in symptoms after 6 months, with the stability of the HRCT score.11 Moreover, a small case series suggests the effectiveness and safety of MMF in many connective tissue diseases, including pSS, ensuring stability or improvement of lung function, especially as a maintenance drug after treatment with RTX.12
In this case, considering the severity of the clinical manifestations at disease onset, we decided to introduce immunosuppressive therapy with MMF to reduce the risk of relapse, saving RTX as possible rescue-therapy.
The number of reports of the early appearance of ILD in patients with misdiagnosed connective tissue diseases, including pSS, is increasing.5 For this reason, we would like to underline the importance of a systematic screening for sicca syndrome as well as other specific symptoms of pSS in patients with newly diagnosed ILD.
Until now, treatment of CTD related acute ILD remains not well defined and often empiric. According to the current evidence, our experience confirms that Rituximab could be a safe and useful treatment for this life-threatening condition.