Benign tracheal stenosis management is still controversial, and there is no international consensus on the best treatment option. Thus, we aimed to look into the history of PITS and the different strategies used in its treatment. The importance of bronchoscopic treatment was also defined, and its effectiveness and safety were assessed.
MethodsRetrospective study of patients diagnosed with PITS, who were referred to the Bronchology Department between January 1996 and December 2016.
ResultsOf 115 patients enrolled (mean age 48.5±17.6 years, 53% males), 66.1% had complex stenosis. The most common causes of intubation were respiratory (29.9%), neurological (26.8%) and surgical (19.6%). Complex stenosis was caused by longer intubation, and was more frequent among previously tracheostomized patients. The most common location was the upper third of trachea (60.9%). Most cases were initially treated by interventional bronchoscopy, and although serial dilations were effective in some complex PITS, a higher proportion of simple stenosis was successfully managed with this treatment option. Long-term recurrence after serial dilation was observed in 25.0% of cases. Stent placement was required (19.1%) only for complex PITS. Stent-related complications were frequent (61.9%) and linked to the stenting time (p<0.001). Overall, there were no procedure-related complications. Surgical intervention was also performed (30.0%), always with complex PITS. Post-surgical recurrences were observed in 24.2% of cases.
ConclusionsInterventional bronchoscopy is an efficient and safe modality in PITS management. Further studies are needed for better classification and improved knowledge of PITS pathogenesis, and to achieve international consensus of definition to guide clinicians in their practice.
Benign tracheal stenosis is a debilitating and potentially life-threatening condition that, in most cases, is caused by iatrogenic events as a result of endotracheal intubation and tracheostomy.1 The management of post-intubation tracheal stenosis (PITS) is still controversial and there is no international consensus about the best treatment option.2 Surgical resection of the affected trachea followed by anastomosis is considered the definitive treatment for this condition.1–3 Although surgery is still considered the first treatment option, especially in complex stenosis,2,4 interventional bronchoscopy has shown promising results in selected patients with simple stenosis.5 In fact, some authors have proposed bronchoscopic treatment as an alternative to surgery for patients who were not eligible and those awaiting surgery.2,6,7 Moreover, recent data demonstrated the crucial role of bronchoscopic approach in the management of benign tracheal stenosis and suggests that, after a correct stenosis classification and careful patient selection, this treatment modality may represent a definitive strategy in most cases.4,8,9
In this context, the aim of the present study was to investigate how PITS has evolved and the several strategies used in its treatment, to define the importance of bronchoscopic treatment and to evaluate its effectiveness and safety among PITS patients.
Materials and methodsA retrospective study was performed including patients diagnosed with PITS referred to the Bronchology Department of Centro Hospitalar e Universitário de São João (CHUSJ), a tertiary hospital in Porto, Portugal between January 1996 and December 2016. Demographic and clinical data were reviewed. This study had the approval of the Ethics Committee of CHUSJ.
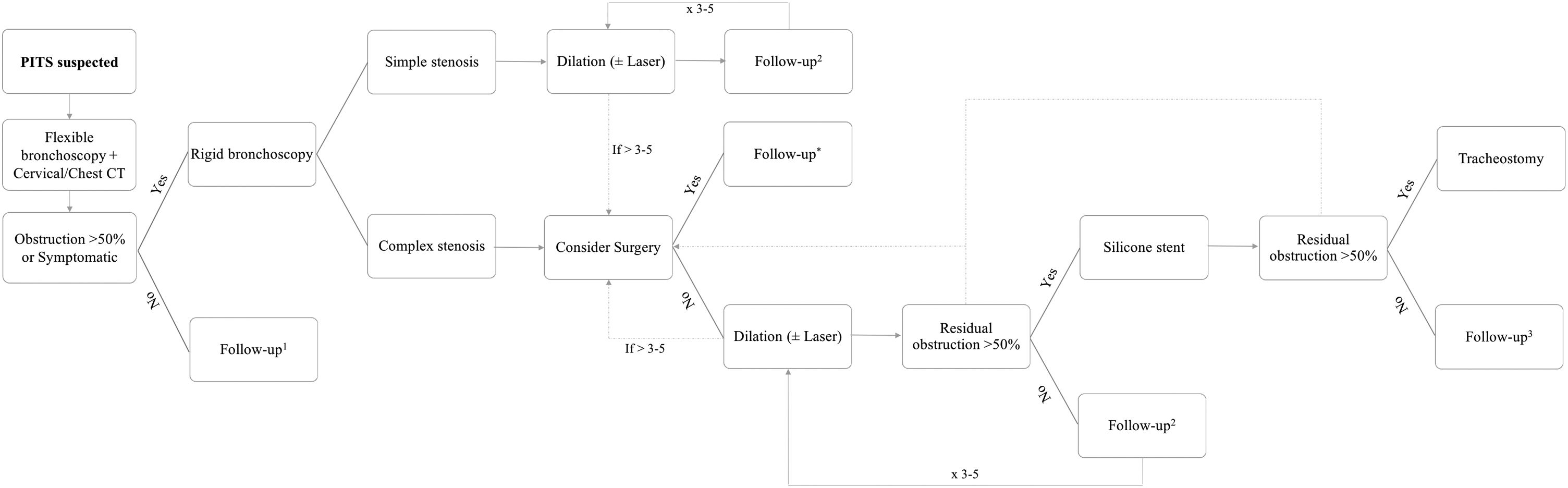
PITS evaluation and treatment decisionWhen a PITS was suspected (Fig. 1), patients underwent a flexible bronchoscopy to diagnose and evaluate stenosis characteristics, which are location, vertical extension and severity of obstruction and stenosis classification as simple or complex. A thoracic and cervical computer tomography (CT) was performed in non-emergency cases to help classification. Simple PITS were defined as web-like lesions with <1cm in vertical extension, whereas complex ones were lesions with >1cm in extension and/or with tracheomalacia or cartilage involvement.10
After stenosis assessment, symptomatic patients or those with >50% of airway obstruction were intubated with a rigid bronchoscope (RB) (Karl Storz, Tuttlingen, Germany). Patients with simple PITS underwent mechanical dilation using increasing RB sizes as the first treatment option, and a balloon, when appropriate. A diode laser (Multidiode endolaser 30, Intermedic Arfran, Barcelona, Spain) was used in selected cases for granulation tissue ablation and to assist mechanical dilation using the spare mucosal technique.5
Patients with complex PITS were always considered for surgical resection of the affected trachea and further end-to-end anastomosis.11 Patients ineligible for surgery (severe comorbidities or more than 4cm length) or symptomatic patients waiting for surgery underwent bronchoscopic treatment. In complex PITS, if dilation was ineffective (≥50% of obstruction after procedure), a silicone stent (Novatech, La Ciotat, France) was placed as described by Dumon.12
Patients were regularly and exclusively followed-up in our department, according to their clinical status (Fig. 1), and were able to contact the team if symptomatic. In every visit a clinical assessment was performed and if symptoms or signs related with stenosis recurrence developed, a new bronchoscopic evaluation and appropriate treatment were performed. In patients whose stenosis was managed with a silicone stent, the stent removal decision was individualized for each case according to subsequent bronchoscopic evaluations. When stent-related complications occurred and the airway patency was reasonable (at least less than 50% of residual obstruction) after stent removal, the patient remained without stent. Otherwise, a new silicone stent was placed. The stenosis was considered solved when there was no recurrence after 12 months from the last intervention. After that, if any additional intervention was necessary, it was considered a long-term recurrence.4
Statistical analysisCategorical and continuous variables were described as absolute (n) and relative frequencies, and as mean and standard deviation (SD), or median, and minimum and maximum values, when appropriate, respectively. Mann–Whitney test or t-test for independent samples were used to test a hypothesis on continuous variables, while for categorical variables, the chi-square and Fisher’s exact tests were applied, as appropriate. Statistical Package for the Social Sciences (SPSS, IBM Corp.) software, v. 25.0, was used for all statistical analysis, with an alpha set at 0.05.
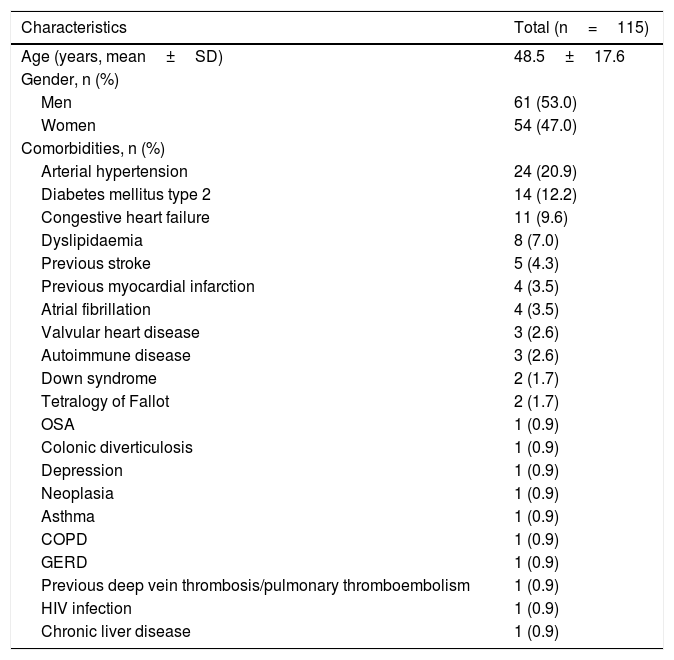
ResultsFrom a total of 115 patients enrolled in this study (Table 1), with a mean of 48.5±17.6 years, 53% (n=61) were males and 47% (n=54) were females. The median follow-up time was 23 months (range, 0.2–130.8). Comorbidities were present in 46 (40%) patients.
Patients’ characteristics.
| Characteristics | Total (n=115) |
|---|---|
| Age (years, mean±SD) | 48.5±17.6 |
| Gender, n (%) | |
| Men | 61 (53.0) |
| Women | 54 (47.0) |
| Comorbidities, n (%) | |
| Arterial hypertension | 24 (20.9) |
| Diabetes mellitus type 2 | 14 (12.2) |
| Congestive heart failure | 11 (9.6) |
| Dyslipidaemia | 8 (7.0) |
| Previous stroke | 5 (4.3) |
| Previous myocardial infarction | 4 (3.5) |
| Atrial fibrillation | 4 (3.5) |
| Valvular heart disease | 3 (2.6) |
| Autoimmune disease | 3 (2.6) |
| Down syndrome | 2 (1.7) |
| Tetralogy of Fallot | 2 (1.7) |
| OSA | 1 (0.9) |
| Colonic diverticulosis | 1 (0.9) |
| Depression | 1 (0.9) |
| Neoplasia | 1 (0.9) |
| Asthma | 1 (0.9) |
| COPD | 1 (0.9) |
| GERD | 1 (0.9) |
| Previous deep vein thrombosis/pulmonary thromboembolism | 1 (0.9) |
| HIV infection | 1 (0.9) |
| Chronic liver disease | 1 (0.9) |
COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; HIV, human immunodeficiency virus; OSA, obstructive sleep apnoea.
Stenosis was classified as follows, 33.9% (n=39) were categorized as simple and 66.1% (n=76) as complex. Gender was the only patient-related characteristic that was associated to stenosis type (p=0.008), simple PITS was more frequent in women (n=25 vs. n=14) and complex PITS more frequent in men (n=47 vs. n=29). This finding was independent of other patients or stenosis characteristics.
With regards to stenosis causes and features (Table 2), median intubation time was 15 days (range, 1.0–118.0) and the most common causes of intubation were respiratory (n=29, 29.9%), neurological (n=26, 26.8%) and surgical (n=19, 19.6%). Thirty-three (28.7%) patients had been previously tracheostomized. Complex stenosis was caused by longer intubations compared with simple ones (16.5 vs 10.5 days, p=0.011) and was more frequent among previously tracheostomized patients (34.2% vs 17.9%, p=0.068). The most common location was the upper third of trachea (60.9%, n=70), with a stenosis extension and severity of 2.0cm (range 0.5–6.0) and 80% (range 20–100), respectively. Specifically, complex stenosis had a median 2.6 (range 1.0–6.0) cm of vertical extension.
PITS characteristics and treatment strategies.
| PITS characteristics | Total | Simple | Complex | p Value |
|---|---|---|---|---|
| n=115 | n=39 | n=76 | ||
| Reason for intubation, n (%) | 0.197 | |||
| Respiratory | 29 (29.9) | 6 (15.4) | 23 (30.3) | |
| Surgical | 19 (19.6) | 8 (20.5) | 11 (14.5) | |
| Neurological | 26 (26.8) | 12 (30.8) | 14 (18.4) | |
| Cardiac | 10 (10.3) | 2 (5.1) | 8 (10.5) | |
| Toxic | 1 (1.0) | 1 (2.6) | 0 | |
| Trauma | 8 (8.2) | 2 (5.1) | 6 (7.9) | |
| Burns | 1 (1.0) | 0 | 1 (1.3) | |
| Infectious | 3 (3.1) | 0 | 3 (3.9) | |
| Intubation length, days (median, min–max) | 15.0 (1–118) | 10.5 (1–47) | 16.5 (1–118) | 0.011 |
| Previous tracheostomy, n (%) | 33 (28.7) | 7 (17.9) | 26 (34.2) | 0.068 |
| Location, n (%) | 0.652 | |||
| Subglottic | 25 (21.7) | 11 (28.2) | 14 (18.4) | |
| Upper third of trachea | 70 (60.9) | 21 (53.8) | 49 (64.5) | |
| Middle third of trachea | 14 (12.2) | 5 (37.5) | 9 (11.8) | |
| Lower third of trachea | 6 (5.2) | 2 (12.8) | 4 (5.3) | |
| Severity, % (median, min–max) | 80 (20–100) | 80 (20–100) | 80 (40–100) | 0.066 |
| PITS treatment strategies | Total | Simple | Complex | p Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Serial dilations alone | 52 (47.3) | 35 (67.3) | 17 (32.7) | <0.001 |
| Laser | 7 (13.5) | 6 (17.1) | 1 (5.9) | 0.264 |
| Long-term recurrence | 13 (25.0) | 7 (20.0) | 6 (35.3) | 0.232 |
| Stent | 21 (19.1) | 0 | 21 (100) | <0.001 |
| Stent as 1st strategy | 8 (38.1) | 0 | 8 (38.1) | – |
| Stent removal | 14 (66.7) | 0 | 14 (66.7) | – |
| Long-term recurrence | 7 (33.3) | – | 7 (33.3) | – |
| Stent duration, months (median, min–max) | 21.2 (0.03–71.3) | – | 21.2 (0.03–71.3) | – |
| Surgery | 33 (30.0) | 0 | 33 (100) | <0.001 |
| Surgery as 1st strategy | 4 (12.1) | 0 | 4 (12.1) | – |
| Long-term recurrence | 8 (24.2) | – | 8 (24.2) | – |
| Tracheostomy | 6 (5.5) | 0 | 6 (100) | 0.062 |
Bold: p<0.05.
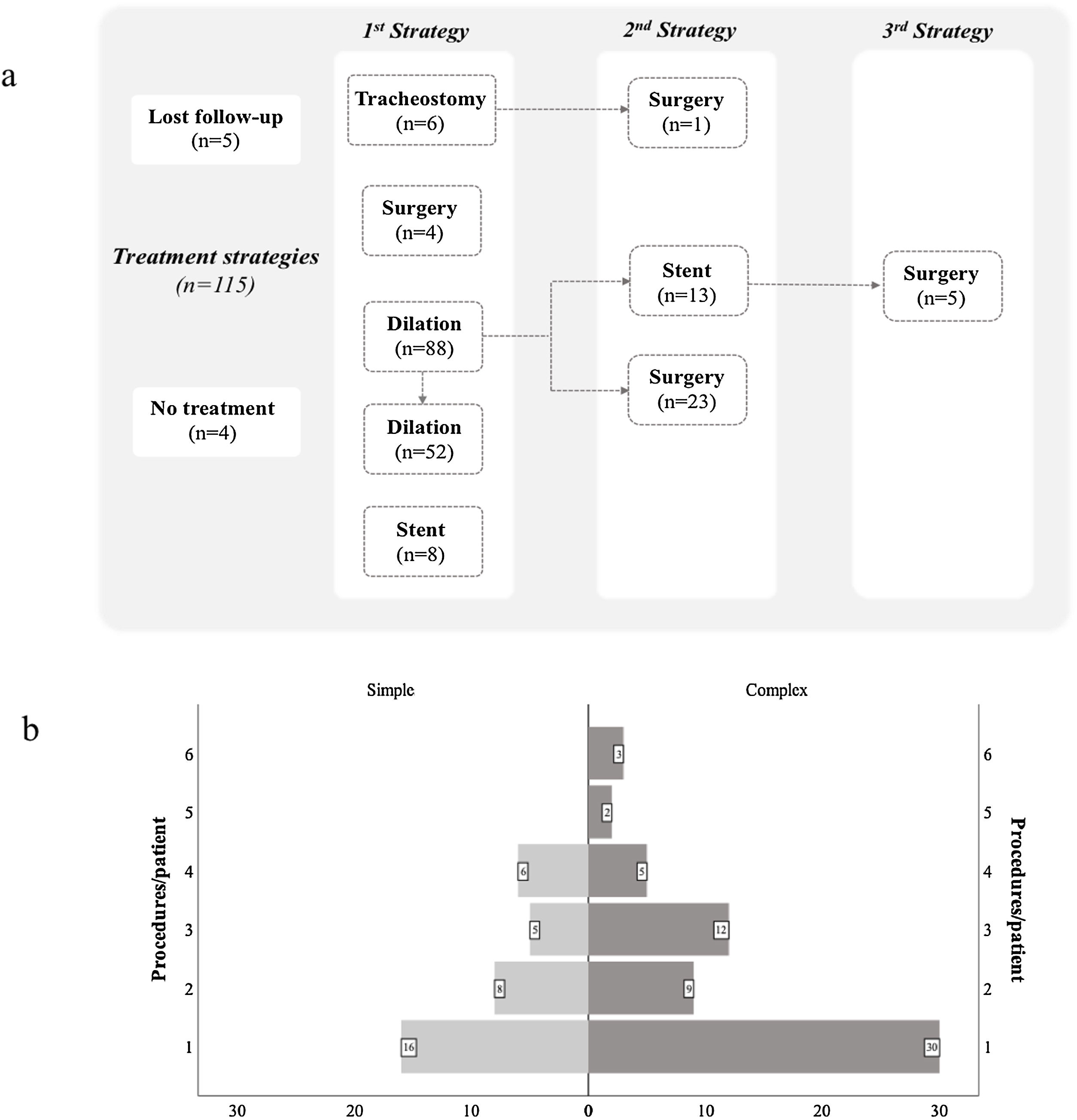
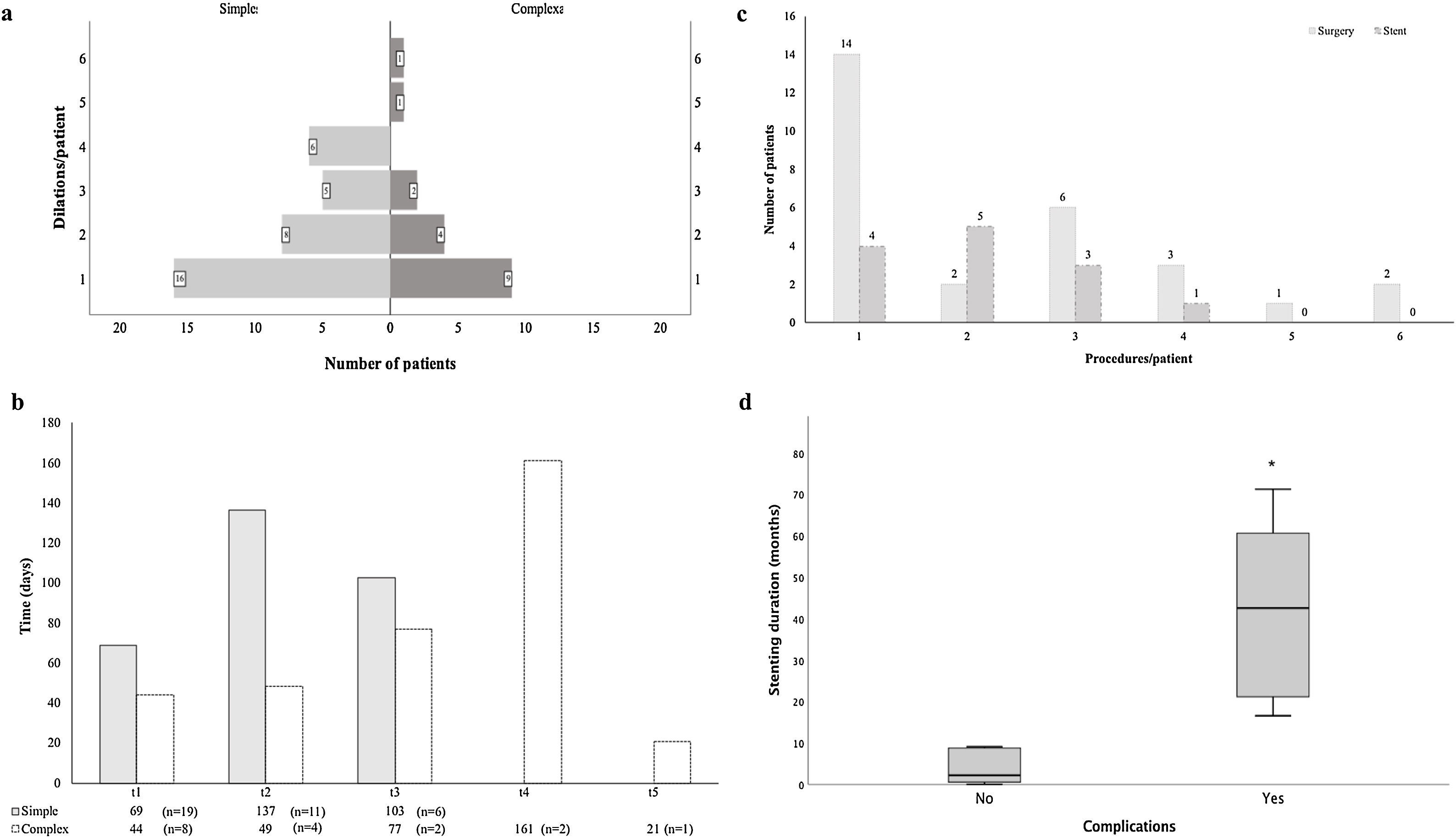
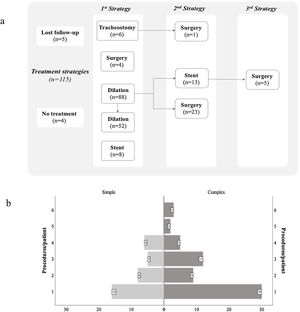
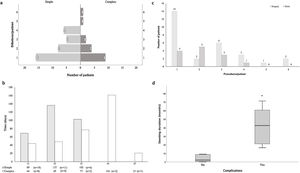
Several treatment strategies and bronchoscopic procedures were necessary for PITS management (Fig. 2 and Table 2). Five patients were followed-up in other centre, even though the first bronchoscopic treatment was performed in our centre as an emergency procedure. The majority of cases were initially treated with a bronchoscopic procedure (n=96), with a median 2.0 procedures (range 1.0–6.0) per patient. Four patients with simple stenosis had no significant obstruction and treatment was not necessary. The remainder (n=35) were successfully treated with serial dilations alone. Despite serial dilations being effective in treating some complex PITS (n=17, 23.9%), a greater proportion of simple stenosis was managed successfully with this treatment option (67.3% vs 32.7%, p<0.001). Laser was used in 13.5% of cases. The number of dilations per patient was 2.0 (range 1–6) and the median time between dilations was 50 (range 14.0–327.5) and 30.6 (range 8.0–154.0) days, respectively, for simple and complex stenosis, with no statistically significant differences (p=0.144) between groups (Fig. 3a and b). Procedure-related complications were not observed. Globally, 25.0% of cases treated with serial dilations presented a long-term recurrence; these recurrences were independent of the stenosis type. These cases consisted of seven simple and six complex stenoses and were successfully managed with additional mechanical dilations.
Complex PITS required other treatment strategies. Stent placement was necessary in 21 (19.1%) patients, it was the first procedure in 8 cases. In the remainder (n=13), mechanical dilation was unsuccessfully attempted prior to stent placement, with a median of 2.0 (range 1–4) dilations per patient (Fig. 3c). The stent was removed in most of cases (n=14), with a median stent duration of 21.8 (range 0.03–69.2) months. Despite no procedure-related complications, long-term stent-related complications were registered in 61.9% (n=13), namely mucostasis (n=8), repetitive respiratory infections (n=8), granulation tissue (n=6) and stent migration (n=5). Granulation tissue and stent migrations were also managed by interventional bronchoscopy. Patients who experienced stent-related complications had a longer median stent time compared to those without complications (42.6, range 16.6–71.3 vs 2.2, range 0.03–23.7 months, p<0.001) (Fig. 3d). Half (n=7) the patients whose stent was removed recurred afterwards. The treatment for these recurrences was diverse: three patients underwent mechanical dilation, two were surgically treated and two had a Montgomery T-tube inserted.
Surgery was performed in 30.0% (n=33) of cases, all of them with complex stenosis. The majority needed other treatment options until surgery was performed: more commonly mechanical dilations (n=23), but also stent placement (n=5). A median of 2.0 (range 1–6) had bronchoscopic procedures until the ideal time for surgery (Fig. 3c). Post-surgical recurrences were observed in 24.2% (n=8) of cases. In a subgroup analysis, there were no differences in patient or stenosis characteristics between patients who recurred and those who did not. Several strategies were used to manage post-surgical recurrences: 5 patients had serial mechanical dilations, 1 had a silicone stent placed (removed after 9 months, no further intervention required), 1 was managed with a Montgomery T-tube and another underwent a second resection surgery. Five patients with complex PITS were definitely tracheostomized. One case was first tracheostomized in emergency contextand then surgery was performed later.
DiscussionBenign tracheal stenosis is a rare but potentially fatal condition when acute. Once established it may cause a significant impact on patient quality of life. At present, there are no international guidelines for PITS management.2 In general, surgical treatment is considered the gold standard for PITS.1–3 However, there is evidence that interventional bronchoscopy may have a role in the management of this condition, not only in inoperable patients or as a bridge to surgery,2,6,7 but also in selected patients as first treatment after a correct stenosis evaluation.4,8,9 We aimed to demonstrate the role of interventional bronchoscopy in treating PITS and assess its efficacy and safety. To better understand PITS pathogenesis, we also analysed patients and stenosis characteristics.
Gender correlated with stenosis type, simple PITS was more frequent in women and complex more frequent in men. In a series of 209 patients with benign tracheal stenosis, although statistical significance was not presented by the authors, male/female ratio was reasonably similar between both types of stenosis.4 To the best of our knowledge, there is no similar data reported in other studies. We suggest that inflammation and scarring may vary with individual patients’ characteristics.
Patients with longer intubation time and prior tracheostomy tended to develop a greater proportion of complex PITS. An increased degree of tracheal wall injury promoted by either prolonged damage stimulus or presence of a second injury element, such as tracheostomy cannula, might explain these findings. It has been proposed that, while PITS typically result from an ischaemic lesion caused by cuff pressure followed by scar contracture,13 post-tracheostomy tracheal stenosis lesions normally imply granulation tissue formation and scar contracture. Besides, additional cartilage damage from tracheal tube attachment at stoma site may occur,14 increasing damage to tracheal wall structures. A retrospective study showed that post-tracheostomy tracheal stenosis patients had more complicated stenosis than those with post-intubation ones.15 Up to 14% of patients requiring mechanical ventilation when admitted to the Intensive Care Unit (UCI) needed long periods of weaning and tracheostomy.16 According to our data, difficult-to-wean patients may be at higher risk of developing complex tracheal stenosis. Evidence has shown that non-invasive ventilation (NIV) may be helpful during the weaning process in specific cases.17–19 A prospective study suggested that NIV may be helpful in achieving weaning in critically ill chronic patients who have hypercapnia after 24h of spontaneous breathing or are unable to increase the duration of spontaneous breathing beyond 18h.20 Similarly, a more recent prospective study showed that in tracheostomised patients partially dependent on mechanical ventilation, decannulation is feasible and safe after switching to NIV.21 Thus, combined NIV/decannulation protocols may represent a way to decrease the risk of developing tracheal stenosis in these patients, improving their quality of life. Furthermore, in the same study, upper airway endoscopy of these patients revealed a preserved respiratory space, with 30% of patients showing moderate mucosal introflection in tracheal cannula convexity and less than 10% had excessive dynamic airway collapse (EDAC) without tracheomalacia.21 Although not analysed in our study, EDAC is a distinct type of airway obstruction, since there is no fixed stenosis, but a dynamic obstruction. Bronchoscopy plays an important role in identifying EDAC and its treatment includes non-invasive positive pressure ventilation, probably acting as a “pneumatic stent”22,23 or, in severe cases, a stent trial to assess whether the patient is a candidate for tracheobronchoplasty and long-term stent placement in selected inoperable patients should be considered.24 As with other conditions associated with upper airway obstructive events, such as obstructive sleep apnoea (OSAS), neuromuscular diseases, exercise-induced laryngeal obstruction and bulbar dysfunction,25 endoscopic evaluation during ventilation titration may be useful in reducing expiratory airway collapse to less than 50%.23
PITS management is complex and most patients need more than one procedure to achieve stabilization. For complex stenosis, several treatment options are necessary, and a combination has been shown to be the best strategy in most cases. Bronchoscopic approach is a safe and less invasive option which is able to manage most cases. According to published data, only a minority of patients experience complications related to rigid bronchoscopy, the diagnosis of benign stenosis being less prone to complications compared to malignant disease or foreign bodies.26 In this study, serial dilations alone were effective in all simple PITS, as previously reported.4,5,9,27
Regarding complex PITS, 23.9% of cases were successfully treated with serial dilations alone, confirming that selected complex stenosis can be managed only with mechanical dilations, as previously described.8 Also, according to other series,4,8,9,28 most cases were treated with 1–3 dilations. In some extreme cases, as in 2 patients with complex stenosis, >3 serial dilations were performed. Excluding these two patients with complex PITS, for whom high subglottic location precluded stent placement, most cases were managed with an acceptable number of dilations. We also present some cases of simple PITS with >4 dilations. Unlike our study, most authors reported stent placement in these cases.2,4,8,9 Instead, we avoided silicone stent placement in simple stenosis given the high rate of complications. Our practice resulted in a maximum of 4 dilations/patient to achieve stabilization.
Despite the high number of procedures in a few cases, stenosis stabilization was achieved by serial dilations alone in most of them, as can be seen by the low rate of long-term recurrences. In general, as the number of dilations increases so does the time until the next dilation, demonstrating stenosis stabilization along serial interventions. The number of dilations, time between procedures and even long-term recurrences did not differ, suggesting that complex PITS are a heterogeneous group that responds distinctly to different treatment options. This might be related to stenosis characteristics, such as the degree of cartilage involvement and tracheomalacia. We believe that complex PITS, with lower grade of cartilage involvement or tracheomalacia, may behave similarly to simple PITS and, therefore, be treated as such.
Silicone stents may be used not only as a bridge to surgery, but also as a definitive long-term treatment of selected, inoperable stenosis.29,30 Compared to other studies, a lower proportion of cases with complex PITS placed a stent in our series.4,8,9 Instead, up to four dilations were performed before stent placement. Although it is known that stenosis <1cm in vertical extension and those without malacia are more likely to be successfully treated by stent placement,22 in our practice, we consider stent placement only for highly unstable stenosis, impossible to manage with serial dilations due to the high rate of stent-related complications. The exact timing for stent removal and, specifically in complex stenosis, the time of cartilage regeneration is unknown; thus, long-term stenting may be necessary.22 Stenting time in our series was in line with the previously reported data.4,8,31 Stent removal was impossible in 7 cases due to absence of alternative strategies. Stent-related complications, associated with longer stenting times, were frequent, but comparable to the previously reported data.8,9,32 Hence, if long-term palliative silicone stents are necessary, these patients demand closer clinical and bronchoscopic surveillance. Recurrences after stent removal were disappointing. Data published are conflicting, with some studies reporting a lower proportion of recurrences4,33 and others similar findings.30,34 It is worth noting that Montgomery T-tubes are effective, either as an alternative to surgery or when other types of stents are unsuccessful among complex PITS patients.35
Surgery, as first treatment option, was performed only in a minority of cases. This data agrees with previous studies4,9 and supports the fact that interventional bronchoscopy may represent an alternative.2,4,6,7,9 Frequently, treatment of comorbidities is necessary to optimize surgical conditions and meanwhile, interventional bronchoscopy may be the only way to relief symptoms. Moreover, high subglottic stenosis as well as long vertical extent (>4–6cm) represent limitations to surgery.22 Post-surgical restenosis was higher than that reported in other studies,36–40 however we found no patients or stenosis features that could explain these results. Given the small sample size of patients undergoing surgery, this analysis may not be representative. However, it seems that surgery improved stenosis characteristics, such as the degree of affected cartilage and malacia, as demonstrated by the success of mechanical dilations in most cases and the good results obtained with silicone stent placement. In fact, interventional bronchoscopy plays an important role in these cases, and is often the only way to improve patients’ symptoms.
Data on topical mitomycin C application was not available, despite its rare use. However, the role of topical mitomycin C in PITS is not well-established. Although some retrospective studies reported positive results,41–43 more recently, a randomized controlled trial suggested that its use brings no additional benefit.44
Overall, these data reflect the importance of interventional bronchoscopy in PITS management, demonstrating it to be efficient and safe. The main advantage of interventional bronchoscopy is that it is less invasive, avoids intra-operatory risks and provides treatment of patients with important comorbidities or longer stenosis. The retrospective design of the study limited access to important data and, subsequently, the statistical analysis. Thus, it was not possible to perform an objective measurement of symptoms, pulmonary function or the application of severity assessment tools, and it was impossible to quantify the conclusions about the success rate of interventions. Objective tools are needed to achieve a better classification and improve knowledge about PITS pathogenesis to ensure a correct stratification and treatment decision. Further studies with a larger, prospective and multicentre design are required as well as international consensus to guide clinicians in their practice.
Statement of ethicsThis study was approved by Ethics Committee of Centro Hospitalar e Universitário de São João and conducted in accordance with the Helsinki declaration.
Conflict of interestThe authors declare no conflict of interests.
CRediT authorship contribution statementC. Freitas: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. N. Martins: Investigation, Methodology, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. H. Novais-Bastos: Validation, Visualization, Writing - review & editing. A. Morais: Resources, Validation, Visualization, Writing - review & editing. G. Fernandes: Resources, Validation, Visualization, Writing - review & editing. A. Magalhães: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing.
N. Martins thanks the Portuguese Foundation for Science and Technology (FCT – Portugal) for Strategic project ref. UID/BIM/04293/2013 and “NORTE2020 - Programa Operacional Regional do Norte” (NORTE-01-0145-FEDER-000012).