Cancer treatment during pregnancy is a rare challenge. It is defined as the cancer diagnosed from the first day of childbearing to 1 year postpartum.1 The incidence is approximately 1 in 1000 pregnancies2 and in Europe annually 3000–5000 patients are diagnosed with this condition.3 Cancer is the second leading cause of mortality in women aged 25–39 years.4 The most frequently diagnosed cancers are those more commonly seen during the reproductive age of a woman, particularly breast cancer, cervical cancer, Hodgkin's disease, melanoma and leukemias.5
Worldwide, lung cancer remains the leading cause of cancer incidence and mortality, with 2.1 million new lung cancer cases and 1.8 million deaths predicted in 2018.6 Tabaco use is, by far, the most important risk factor for lung cancer, being responsible for 70% of global lung cancer deaths.7 Although lung cancer counts as one of the most common malignancies, it represents a rare tumour during gestation.8 However, such an association is expected to rise due to delayed pregnancies as well as the increased cigarette consumption by women in developed countries.9 In the literature, there are few cases of lung cancer during pregnancy described. Most of them are non-small cells.10,11
Treating a pregnant woman with cancer requires a delicate balance between maternal benefit and fetal risk.12 The general golden rule in the management of pregnant women with cancer diagnosis is the treatment should not differ between pregnant and not pregnant women, if this is feasible.1 The treatment goals for a pregnant woman are to try to increase survival of the mother, to treat the curable malignant disease of pregnant women and to protect the fetus and the newborn from the ill effects of cancer treatment.
Making a decision can be more difficult when cancer is at an advanced stage at diagnosis and no curative treatment is available. It is necessary to consider various factors related to treatment. The administration of chemotherapy during gestation can cause harmful effects to the fetus and to the mother. For the fetus the detrimental effects include malformations, teratogenesis, mutations, carcinogenesis, organ toxicity and retarded development; for the mother, they include spontaneous abortion and sterility,2 along with all the other adverse effects and current challenges of chemotherapy treatment, in this case in pregnant women.
Given the lack of safety profile studies of the different chemotherapy regimens and target therapies during pregnancy, it is essential to report clinical cases and to share evidence about the use of antineoplastic therapies during the gestation period.11
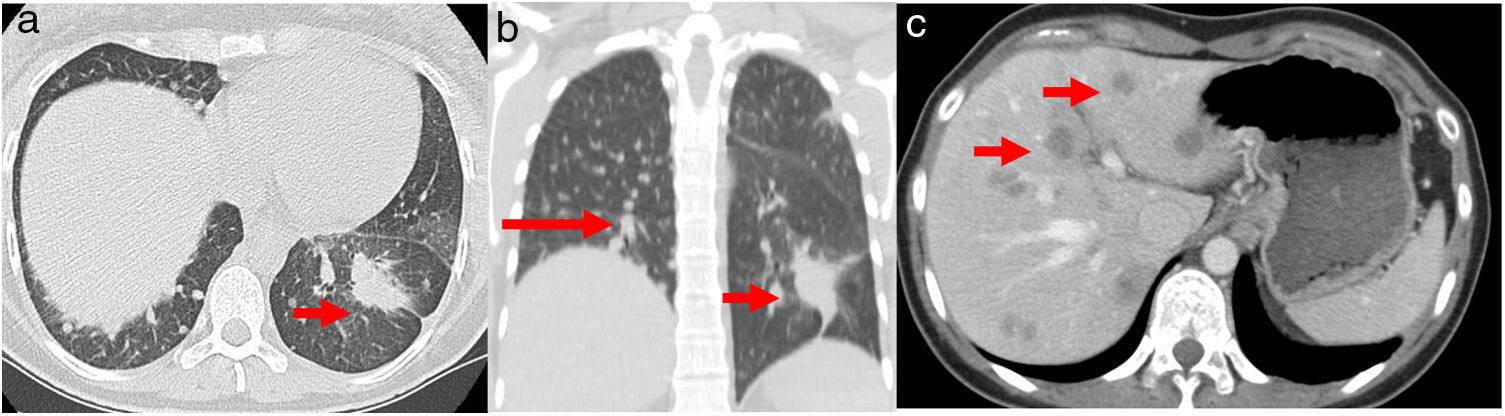
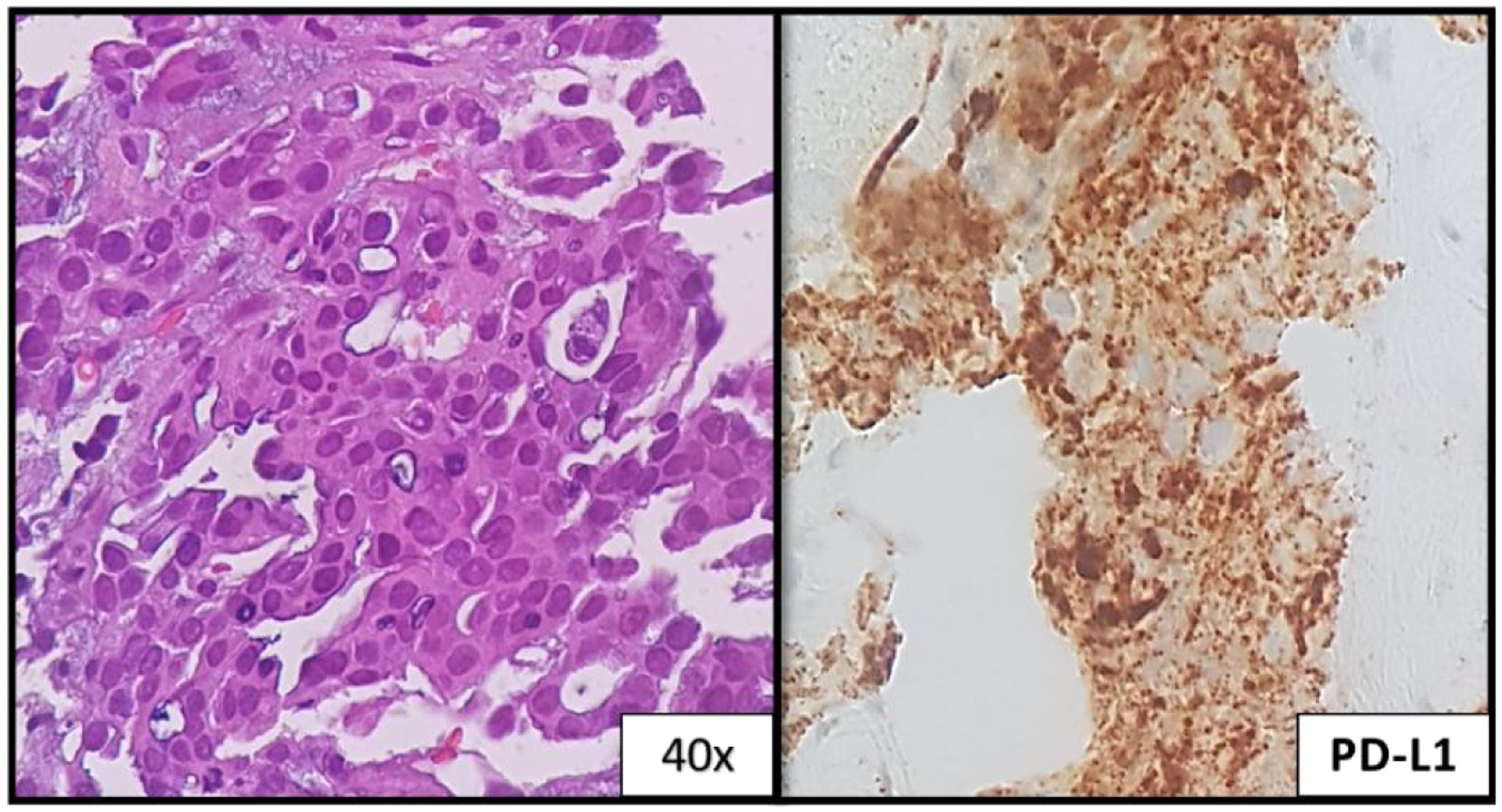
A 32-year-old woman, 27 weeks pregnant, non-smoker, no family history of cancer, was admitted to the emergency department with complaints of dry cough with 2 months of evolution. On examination she had palpable cervical adenopathy. Thoracic CT revealed left lower lobe mass with 4cm, multiple small diffuse round nodules and bilateral mediastinal adenopathies (Fig. 1a and b). Cervical lymph node fine-needle aspiration biopsy revealed adenocarcinoma, consistent with metastatic lung cancer [positive for cytokeratin 7 (CK7) and thyroid transcription factor-1 (TTF1)]. The patient was diagnosed as having primary lung adenocarcinoma, cT4N3M1a, stage IVa disease. To determine the optimal therapeutic strategy, an additional next-generation sequencing (NGS) testing of the tumour specimen was conducted. The test yielded a negative result for mutation of EGFR, ALK rearrangement, ROS1 and BRAF V600E but with presence of KIF5B(15)-RET(12)rearrangement. PD-L1 expression was negative.
(a) Chest axial CT; (b) non-contrasting coronal CT shows left lower lung mass (arrow) and multiple small diffuse round nodules (long arrow) at diagnosis; (c) contrast axial abdominal CT shows multiple hypo-enhanced solid liver nodules according to diffuse liver metastases (arrows) following hepatic progression.
She initiated chemotherapy at 28 weeks+6 days of gestation with carboplatin and paclitaxel scheme (carboplatin AUC5 D1, paclitaxel 80mg/m2 D1, D8 and D15 4/4 weeks). At 30 weeks of gestation she developed a respiratory infection and a caesarean section was performed. The patient was delivered of a normal female newborn whose birth weight was 1070g. The Apgar scores were 7, 8, and 9 at 1, 5, and 10min, respectively. The placenta had no disease by gross and pathological examination. She had pulmonary progression and started treatment according to the cisplatin and pemetrexed regimen having completed 4 cycles followed by maintenance with pemetrexed. After 4 cycles, she had liver biopsy-proven progression that revealed metastatic lung adenocarcinoma, now with PD-L1>50% (Figs. 1c and 2). She initiated pembrolizumab 2mg/kg with hepatic progression after 3 cycles. Vandetanib was proposed, but given hepatic function worsening, treatment with gemcitabine was initiated. As complications, a deep venous thrombosis was diagnosed, and low molecular weight heparin was started. Later she had an ischemic stroke, worsening of the general state and suspension of systemic treatment. She died about 12 months after diagnosis. The baby is healthy and has a normal development for her age.
In pregnant women with lung cancer, adenocarcinoma is the most common, accounting for 80% of cases. More than 97% of the published cases are diagnosed with locally advanced or metastatic disease,13 largely due to diagnostic delays due to attribution of symptoms to other etiologies and efforts to ensure fetal well-being. Approximately, 20–30% of malignant tumours occur in women younger than 45 years14 as with our patient.
Full-term pregnancy (≥37 weeks) should be always the goal, according to all current treatment guidelines, since prematurity influences the emotional and cognitive development of children.15 In pregnant women, cancer is often diagnosed at a later stage than in nonpregnant females. This fact might explain the poorer outcome of pregnant patients with cancer reported in some publications. However, the pathological characteristics and outcomes of patients in whom cancer is diagnosed during pregnancy are comparable to those of age- and stage matched nonpregnant patients with cancer.16
Metastatic transmission to the products of conception is a rare phenomenon, but 11 cases of placental metastases and 3 cases of fetal metastases were reported secondary to maternal lung cancer.17–19 The most likely way for dissemination is through the hematogenous route. The rarity of this dissemination is probably due to the placental barrier and the fetal immune system.20 The placenta of our patient was normal.
The main concerns for these patients are to choose the proper treatment and the overall survival from the disease. In the meantime, it is of great importance to take into consideration the consequences of the chemotherapeutic drugs on the developing fetus, as well as the long-term complications after in utero exposure to anticancer therapy.1
Nowadays we need to focus on those tumours that require systemic treatment since it is now widely acknowledged that surgery may be administered to pregnant women with no damage to the foetus in any phase of pregnancy. Chemotherapy is the treatment of choice in most cases of lung cancer in pregnancy.14 Drugs with a molecular weight of less than 500–600 cross the placenta, whereas those with molecular weights greater than 1000 are reported to cross poorly.3,21 Most traditional antineoplastic agents have a weight below 400, resulting in potential chemotherapy exposure to the fetus. Congenital malformations can occur in approximately 20% of cases if cytotoxic anticancer drugs are administered during the first trimester and thus should be avoided.22 Among the therapeutic drugs, antimetabolites (aminopterin, methotrexate, 5-fluorouracil, arabinosyl cytosine) and alkylating agents (busulfan, cyclophosphamide, chlorambucil) are the most common drugs reported to induce malformation or to exert teratogenic effects. Vinca alkaloids and antibiotics seem to have no effect on the fetus; however, cisplatin is implicated in growth restriction and hearing loss, whereas etoposide is implicated in pancytopenia.2 There are several published case reports with carboplatin plus paclitaxel treatment during pregnancy.
Since teratogenic effects of chemotherapy have been described, we can assume that at least a fraction of these drugs pass the placenta.23 The teratogenic potential of any drug depends on a variety of factors that include the extent of its placental transfer, the dose administered, the duration of exposure, the genetic variability in drug metabolism of the mother and fetus, and timing of exposure. Hence, pregnancy termination should be considered in pregnant patients with cancer who need chemotherapy administration in the first trimester.24,25 Based on multiple studies, giving chemotherapy after the first trimester is safer; however, there is a relatively higher risk of premature rupture of membranes, intrauterine growth restriction and premature labour.
The pharmacology of various anti-cancer drugs may be altered by the normal physiological changes that occur during pregnancy, such as increased plasma volume, enhanced renal and hepatic elimination, and decreased albumin concentration.26 However, it is still not clear whether pregnant women should be treated with different doses of chemotherapy, and no studies have addressed the effectiveness of treatment regimens in pregnancy.22
There are no data evaluating molecular and genomic characteristics for lung cancer in pregnant women. As in pregnant patients the rate of lung cancer in non-smoking females can reach more than 40% in published cases one might assume a higher incidence of cancers with targetable molecular alterations (e.g., sensitizing EGFR mutations, anaplastic lymphoma receptor tyrosine kinase gene [ALK] translocations). Dagogo-Jack et al. reported 8 cases of lung cancer during pregnancy: six patients had an ALK translocation, and two patients showed a sensitizing EGFR mutation.27 The RET rearrangement of our patient is not described in the literature.
The most widely reported scheme of chemotherapy in pregnant woman with lung cancer is carboplatin and paclitaxel, which has been reported as well in ovarian cancer during pregnancy with acceptable toxicity. The weekly application of paclitaxel allows a lower peak plasma concentration of the drug resulting in lower maternal toxicity and possible lower placental transfer. Carboplatin seems slightly safer than cisplatin during pregnancy and hence was favoured.12 Our patient was treated according to this scheme with optimal tolerance and without significant toxicity.
The vinca alkaloids vincristine, vinblastine and vinorelbine have been used safely after the first trimester. Three cases of vinorelbine use in pregnancy have been reported, all three infants are growing normally and were born without congenital abnormalities. The use of gemcitabine and pemetrexed should be discouraged due to the lack of data.
It should be clearly stated that there are insufficient data for the proper management of pregnant women with cancer; guidelines are mainly based on data coming from small retrospective studies or case series with limited follow-up.28 In these cases, the multidisciplinary discussion for therapeutic decision making becomes even more important. Maternal outcome is very poor. Post-partum maternal median survival is generally poor, and the majority are known to have died within 1 year after delivery.18 Despite all the advances in lung cancer in recent years, the outcomes in this subgroup remain scarce with survivals that do not exceed 12 months, as with this patient.
The diagnosis of lung cancer during pregnancy is rare, and often made at an advanced stage since symptoms can be easily attributed to other causes and there is the fear of carrying out diagnostic tests during pregnancy. The most studied protocols in the treatment of lung cancer during pregnancy include platinum and paclitaxel.
In a subject in which there are no randomized controlled trials, where the number of reported clinical cases is low, evidence is scarce. The clinical decision is based on knowledge of treatment in non-pregnant patients, the few cases described in the literature, the assessment of potential toxicity and the potential benefits of treatment. Decisions should always be made in a multidisciplinary group where obstetricians and neonatology are also present. Multidisciplinary management is essential to provide better outcomes for our patients. It is also important to report more and more cases, as well as the molecular and genetic studies of these tumors to increase the experience and safety of using targeted therapies.
Ethical disclosuresThe submitted document is a review. It does not involve experimentation on animals or humans. The study is not a clinical trial and is not part of a trial. And all clinical case data are referred to in the discussion and conclusions.
Conflict of interestsThe authors declare no conflict of interests.