Nontuberculous mycobacteria (NTM) are considered opportunistic pathogens. They are widely distributed in the environment, and several species are associated with a wide range of infections, most commonly affecting the lung.1–3 The increasing number of immunocompromised patients and predisposing diseases – such as chronic obstructive pulmonary disease (COPD), silicosis and bronchiectasis—as well as the increasing life expectancy of patients with cystic fibrosis, are factors contributing to a rising incidence of NTM disease.2–4 However, patients can develop NTM disease without any apparent underlying cause. This suggests that NTM disease is a multifaceted disease, and the genetic background or environmental exposures could, also, increase susceptibility to infection.1–3 Population-based studies across Europe report a steady increase in NTM isolation and related lung disease over time. In Portugal, epidemiological studies are scarce but are in line with these reports. Two studies, conducted in Lisbon Area, found NTM to account for approximately 12% of all mycobacterial isolates,5,6 and hospital-based research7 showed a definite rise in the number of NTM isolates each year of the study, and 89% of those were found to be clinically significant, fulfilling the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) criteria.2
NTM disease is complex to manage and a significant cause of morbidity and mortality.4 Treatment is long and requires multiple drugs, which can be challenging due to side effects and different resistance profiles.2 Therefore, the treatment success rate remains unsatisfactory.4 Host factors and comorbidities can influence the outcome, and this is rarely covered by studies reported to date or in the current guidelines.
The present study aimed to identify the effect of alcohol consumption on the treatment outcome of NTM disease.
This study collected information from the structured questionnaire filled in by clinicians who initiate treatment for NTM disease and which is stored in the Portuguese National Epidemiological Surveillance Commission for Tuberculosis. We retrospectively reviewed the data from patients with NTM disease from January 2003 to December 2016. All patients met the diagnostic criteria for NTM disease from the ATS/IDSA guidelines. Clinical and socio-demographic data were collected. Alcohol consumption was self-reported in the questionnaire at time of diagnosis and was defined as the presence or absence of consumption. Excluded from the main outcome analysis were patients still in treatment, those who had discontinued the therapy, those lost to follow-up or transferred out.
Treatment outcome of NTM disease was binarily evaluated as a good or bad outcome. Good outcome was defined as culture conversion after initiation of treatment and maintenance of a negative culture for ⩾12 months on treatment. Bad outcome was defined as no culture conversion or as death while being treated for NTM disease. Simple logistic regression models evaluated univariate effects, and multiple logistic regression models identified the simultaneous effects of potential risk factors for a bad outcome. Selection of the final model was based on the backwards algorithm combined with the results from the univariate analysis. The statistical analysis was performed with the R language and software environment for statistical computation [The R Project for Statistical Computing, https://www.r-project.org/, version 3.4.3]. The significance level was set to ≤0.05.
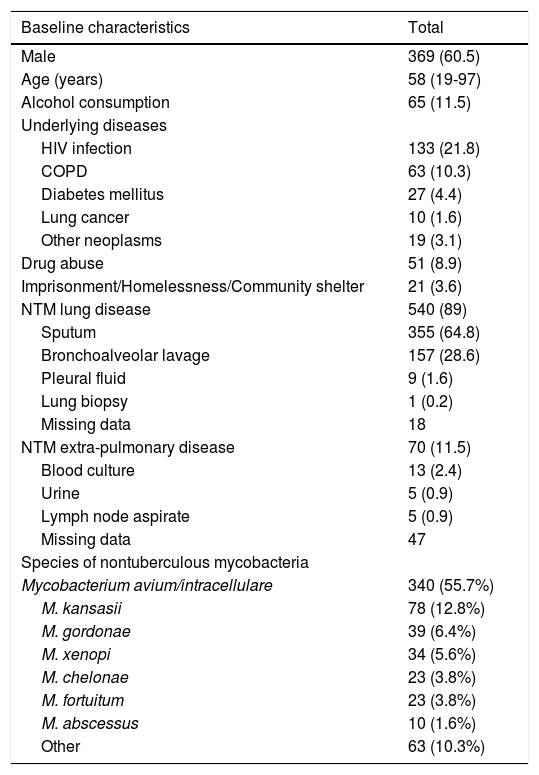
During the study period, 610 patients were eligible. The median age was 58 years (min-max, 19–97 years), and 369 (60%) of the patients were male. The vast majority of patients had NTM lung disease (n = 540, 89%). Alcohol consumption was reported at diagnosis in 65 (11.5%) patients. Table 1 shows the population demographic and clinical characteristics.
Baseline characteristics of the study population - data are presented as n (%) or median (min-max); HIV: human immunodeficiency virus; COPD: chronic obstructive pulmonary disease.
| Baseline characteristics | Total |
|---|---|
| Male | 369 (60.5) |
| Age (years) | 58 (19-97) |
| Alcohol consumption | 65 (11.5) |
| Underlying diseases | |
| HIV infection | 133 (21.8) |
| COPD | 63 (10.3) |
| Diabetes mellitus | 27 (4.4) |
| Lung cancer | 10 (1.6) |
| Other neoplasms | 19 (3.1) |
| Drug abuse | 51 (8.9) |
| Imprisonment/Homelessness/Community shelter | 21 (3.6) |
| NTM lung disease | 540 (89) |
| Sputum | 355 (64.8) |
| Bronchoalveolar lavage | 157 (28.6) |
| Pleural fluid | 9 (1.6) |
| Lung biopsy | 1 (0.2) |
| Missing data | 18 |
| NTM extra-pulmonary disease | 70 (11.5) |
| Blood culture | 13 (2.4) |
| Urine | 5 (0.9) |
| Lymph node aspirate | 5 (0.9) |
| Missing data | 47 |
| Species of nontuberculous mycobacteria | |
| Mycobacterium avium/intracellulare | 340 (55.7%) |
| M. kansasii | 78 (12.8%) |
| M. gordonae | 39 (6.4%) |
| M. xenopi | 34 (5.6%) |
| M. chelonae | 23 (3.8%) |
| M. fortuitum | 23 (3.8%) |
| M. abscessus | 10 (1.6%) |
| Other | 63 (10.3%) |
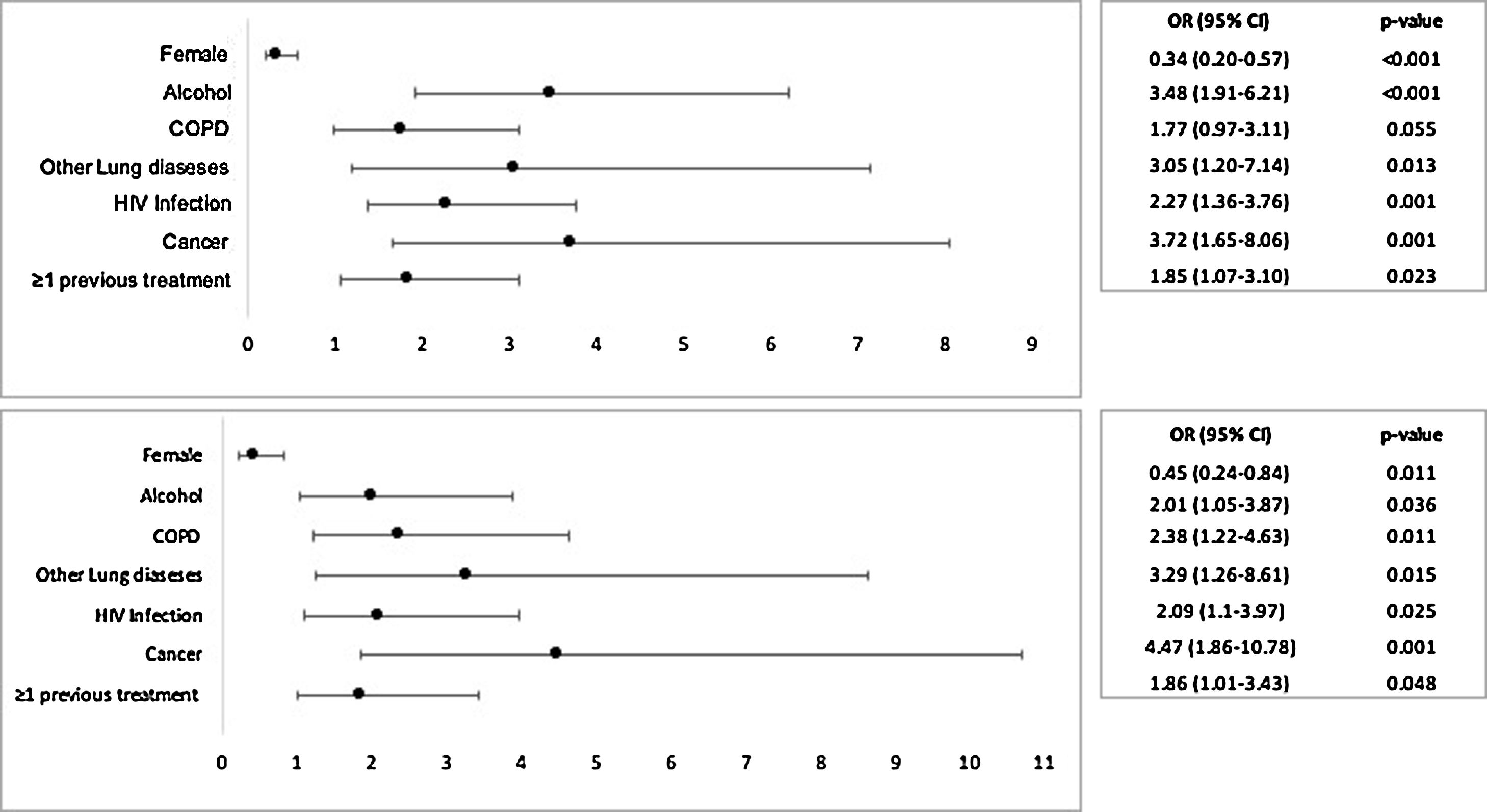
A favourable outcome was achieved in 517 (84.8%) patients, and 93 (15.2%) had an unfavourable outcome. The median total antibiotic treatment duration was longer in patients with a good outcome than in those with a bad outcome (376 vs. 167 days; p < 0.001). The (crude) odds ratio (OR) for a bad outcome among the individuals who declared alcohol consumption was almost 3.5 times the same odds for those that declared they did not drink (95% CI:1.91–6.21, p < 0.001).
The final multiple logistic regression model revealed that alcohol consumption was associated with bad outcome (OR 2.01, 95% CI:1.05–3.87, p < 0.001). This effect was adjusted for history of COPD (OR 2.38, 95%CI: 1.22–4.63 p = 0.011) and other co-morbidities (OR 3.29, 95%CI: 1.26–8.61 p = 0.015), cancer (OR 4.47, 95%CI: 1.86–10.72, p = 0.001), having at least one previous treatment (OR 1.86, 95%CI: 1.01–3.43 p = 0.023) and HIV-infection (OR 2.09, 95%CI: 1.2–3.97 p = 0.001). Female gender was identified as a statistically significant protective factor (OR 0.45, 95%CI: 0.24–0.84 p = 0.001). Fig. 1 represents the Crude OR and Adjusted OR for a bad outcome of NTM disease.
Our results showed that alcohol consumption doubled the odds for a bad outcome, after adjustment for COPD and other comorbidities, cancer, at least one previous treatment, HIV-infection and gender. Among all these confounders, only the female gender was significantly identified as a protective factor.
The ATS/IDSA provides guidelines for the prevention, diagnosis and treatment of NTM infections.2 In the case of NTM disease—in which immediate symptomatic benefits are not always obvious, and treatments can be long and toxic—compliance with therapy is often low. In every patient, the decision to treat NTM disease involves balancing the benefits and the risks of drug toxicity,4 and it is essential to manage the patient’s underlying diseases, such as alcohol dependence, COPD or cancer.
For this study, we relied on self-reported alcohol consumption at diagnosis, which might be the main limitation, as it may not reflect the drinking pattern over the time of the treatment. Other limitations include its retrospective nature, possible incomplete data from the nationwide forms and overestimation of unfavourable outcomes (older patients, more likely to be male, to have alcohol consumption or several comorbidities).
In conclusion, this study supports the hypothesis that alcoholism influences the prognosis of NTM disease. After adjustment for potential risk factors, alcohol consumption was estimated to double the odds of an unsuccessful outcome, underlining the need for close monitoring among patients with alcohol dependencies to improve treatment outcomes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Rita Gaio was partially supported by CMUP (UID/MAT/00144/2013), which is funded by FCT (Portugal) with national (MEC) and European structural funds (FEDER), under the partnership agreement PT2020.