The adipose tissue secretes adipokines and influences the release of inflammatory mediators contributing to a state of low-grade systemic inflammation that may change lung function.
ObjectiveTo correlate levels of adipokines and inflammatory mediators with lung function in individuals with obesity and bronchial asthma symptoms.
Materials and methodsA cross-sectional study, including women with obesity (grade II and III) with symptoms and clinical diagnosis of asthma. Anthropometric measurements (weight, height, BMI), pulmonary function test (spirometry), asthma control test questionnaire, collection of systemic inflammatory markers (blood collection) and pulmonary markers (sputum collection) were collected and were analyzed: IL-6, IL-8, TNF-α, adiponectin, resistin, leptin and C-reactive protein (CRP). The patients were stratified into two groups according to asthma control.
Results80 women were analyzed and 40% had an ACT score greater than or equal to 18 and were classified as “controlled asthma”. More than half of the patients of ACT<18 score obtaining measures of FEV1, PEF and FEF25–75% below and 80% of predicted. There was a significant and negative correlation between IL-6 in the sputum with FVC and FEF25–75% in the group ACT<18 and with FVC and FEV1 in the group ACT≥18.
ConclusionsTherefore, we concluded that the increase of interleukin-6 in the sputum is related to worse pulmonary function even in patients with controlled asthma, especially in the translate airway permeability measures.
The Brazilian Ministry of Health1 research shows that 54% of the Brazilian population over 18 years old are overweight, 57.3% of men and 51.2% of women. The World Health Organization2 shows that in 2016 more than 1.9 billion adults (39%) were overweight and more than 650 million (13%) were obese.
The adipose tissue, being an endocrine organ, secretes adipokines and influences the release of inflammatory mediators and such substances contribute to a low grade systemic inflammation state and can promote directly or systemically changes in pulmonary function.3–5 Increased adipose tissue may also influence the susceptibility to lung infections, increased lung inflammation with environmental exposure, and airway obstruction exacerbation in preexisting lung diseases.6
Beuther et al.7 observed in a meta-analysis that the obese individual is more likely to develop asthma in comparison to eutrophic, and in Melo et al.8 study, asthma prevalence in the obese population was 18.5% in a sample of 363 obese, and in Baltieri et al.9 study, asthma prevalence was 4.6% in a sample of 4791 obese.
In view of the vast metabolic and inflammatory changes that accompany the obese individual, altered pulmonary function is evident in many studies, both by inflammatory and mechanical origin. Studies have therefore shown lung function alteration in the obese individuals without associated prior disease, such as the mechanical changes caused by adipose tissue deposition around the thorax and abdomen, which include reduction of Expiratory Reserve Volume (ERV),10 and may cause areas of atelectasis, especially in the pulmonary bases.11,12 In addition to inflammatory alterations, inflammatory cytokines act on the lung which causes lung function impairment. Such changes are evidenced in the literature by the reduction of Forced Expiratory Volume in the First Second (FEV1), Functional Residual Capacity (FRC), Forced Expiratory Flow in 25–75% (FEF25–75%) and increase in FEV1/FVC (Forced Vital Capacity) ratio.5,13,14
Thus, the aim of the present study was to correlate adipokines levels and inflammatory mediators with lung function in individuals with obesity and bronchial asthma symptoms.
MethodThis is a cross-sectional study conducted at a training hospital, approved by the Research Ethics Committee, registered in the Brazil Platform and with Research Support from the Foundation for Research Support of the State of São Paulo (FAPESP).
SamplingThe study population was included provided they fulfilled the following criteria:
Inclusion criteria
- -
Obesity grade II and III (BMI≥35kg/m2)
- -
Feminine gender
- -
Initiating multiprofessional follow-up at the Institution's Ambulatory Surgery
- -
Manifestation of asthma symptoms and clinical diagnosis according to the Global Initiative for Asthma15 and the Guidelines of the Brazilian Society of Pulmonology and Phthisiology (SBPT) for Asthma Management.16
Exclusion Criteria
- -
Smoking
- -
Cognitive limitations that prevent the understanding of the tests or belonging to the vulnerable groups
- -
Chronic or acute inflammatory diseases, with asthma exception.
1. Pulmonary function test
Spirometry was performed at the Pulmonary Function Laboratory of the institution by trained technicians. EasyOne equipment was used and followed the standards of the American Thoracic Society – ATS and European Respiratory Society – ERS.17 Two maneuvers were performed to assess the pulmonary volume and flow measurements: slow vital capacity (SVC) and forced vital capacity (FVC). The maneuvers were performed until three acceptable and two reproducible curves were obtained, with no more than eight attempts. The values extracted from each maneuver were selected according to Pereira.18 To calculate the predicted values, the equation proposed by Pereira et al.19 for the Brazilian population.
The volunteers were rested for 10min prior to the test and were properly guided in performing the maneuvers.2. Collection and processing of systemic inflammatory profile markers
With the patient at 12h of fasting, the blood was collected in a vacuum collection system (Vacuette®), two dry tubes (9ml) for the serum use.
The collected blood was immediately processed in a centrifuge (Eppendorf® brand – model 5804R) with a rotation of 4500rpm for 20min at 4°C. Serum was stored in a freezer for analysis of adipokines and inflammatory mediators.
3. Collection and processing of local inflammatory profile markers
For patient safety the procedure was performed in a hospital environment and accompanied by a pulmonologist with medication and emergency material available.
First, patients underwent spirometry to determine baseline FEV1. Patients, as long as they were not in asthma exacerbation crises, underwent the sputum induction procedure by administering 10ml of 3% saline solution for 12min with an Ultrasonic Nebulizer Inhaler (Pulmosonic Star – Soniclear® – São Paulo, Brazil – Registration in the MS/ANVISA: 80023140008).
Patients were advised, when they felt the need, to cough and expectorate in available Falcon Tube, having rinsed the mouth earlier. If there was no sputum production the above procedure was repeated with 4% and 5% saline solution. Patients were monitored with pulse oximetry to check for peripheral oxygen saturation and pulmonary auscultation to verify bronchospasm presence and, if necessary, at intervals of each inhalation increment, FEV1 was measured as a safety procedure, and if between 10% and 20%, the previous inhalation was repeated; if there was a fall in FEV1>20% the collection was interrupted. If there was a fall in FEV1<10%, sputum induction was continued20.
If there were no intercurrences, the procedure was interrupted as soon as sufficient sputum sample was available for processing.
The obtained sputum sample was processed immediately using DTT and PBS usually in a ratio of 1:1, up to four times the dilution, vortexed and carried in the bain-marie at 37°C with manual shaking with a pipette for 15–20min. The sample was then centrifuged (Thermo Scientific, Legend Mach 1.6R Centrifuge) with rotation of 1800rpm, temperature of 5°C for 10min. The supernatant was stored in a freezer at −80°C for the analysis of adipokines and inflammatory mediators.
Dosage of inflammatory mediators in the blood and sputumThe levels of IL-6, IL-8, TNF-α, adiponectin, resistin, leptin and C-reactive protein (CRP) were measured following the manufacturer's recommendations by the ELISA method (R&D Systems, CA, USA). The reading was performed on a multi-plate reader (SpectraMax i3, Molecular Devices, CA, USA) at a wavelength of 540nm.
Asthma control test (ACT)The ACT21–23 was applied to verify asthma control. It is a self-administered questionnaire and has five items related to the symptom, use of relief medication and asthma impact in daily activities. Each question includes a score between 1 and 5, with 25 total points representing total control of asthma or remission of symptoms, with a score ≥18 being defined as “controlled asthma”. Although the international validation22 considers a cutoff point greater than or equal to 20, the ACT validation in Brazil23 found a cutoff point of 18 or more to categorize “controlled asthma”, therefore, because it is a study in a Brazilian population, we consider the respective cutoff point. The questionnaire was used as a way of describing the stage of disease control.
Statistical analysisThe data were computed in the SPSS program (Statistical Package for Social Science) v13.0 and to describe the sample profile of the variables the means, standard deviation, minimum and maximum values were extracted. The patients were stratified into two groups according to asthma control, one group with an ACT questionnaire score greater than or equal to 18 (controlled asthma) and another group with an ACT score less than 18 (non-controlled asthma).
To compare the variables of adipokines and inflammatory mediators with the spirometric variables, the Spearman Correlation test was performed and the Coefficient r value was presented. The level of significance adopted for this study was 5%.
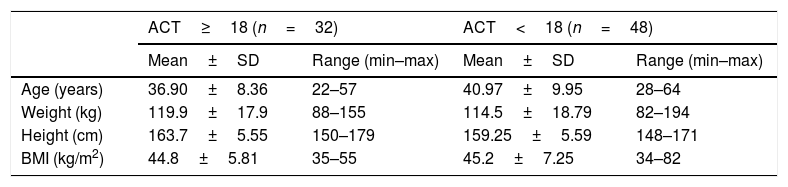
ResultsWe analyzed 80 women who entered the program and the patients who answered the ACT questionnaire to check asthma control 40% had a score greater than or equal to 18 and were classified as “controlled asthma”. The data of the characteristics of each group based on the ACT score are described in Table 1. Of the patients studied, 19.1% had grade II obesity and 80.9% had grade III obesity.
Sample characteristics (n=80).
| ACT≥18 (n=32) | ACT<18 (n=48) | |||
|---|---|---|---|---|
| Mean±SD | Range (min–max) | Mean±SD | Range (min–max) | |
| Age (years) | 36.90±8.36 | 22–57 | 40.97±9.95 | 28–64 |
| Weight (kg) | 119.9±17.9 | 88–155 | 114.5±18.79 | 82–194 |
| Height (cm) | 163.7±5.55 | 150–179 | 159.25±5.59 | 148–171 |
| BMI (kg/m2) | 44.8±5.81 | 35–55 | 45.2±7.25 | 34–82 |
ACT: asthma control test; SD: standard deviation.
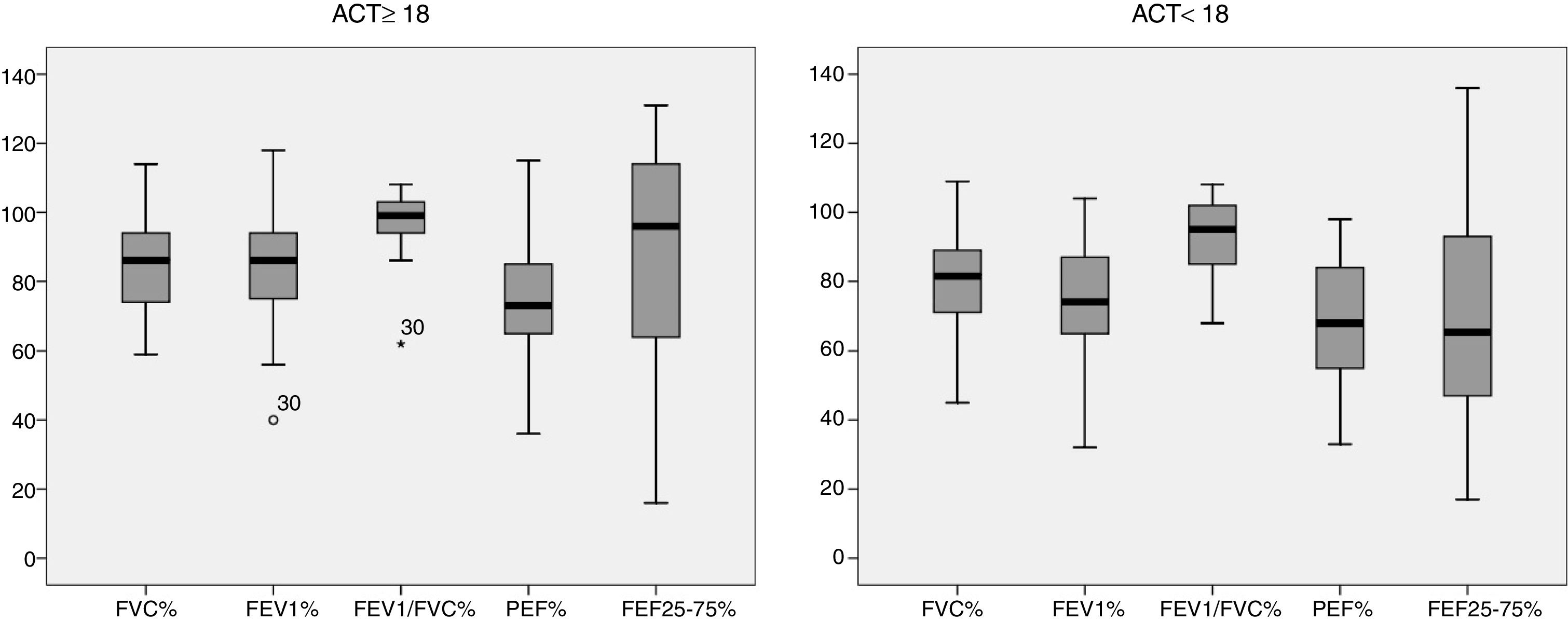
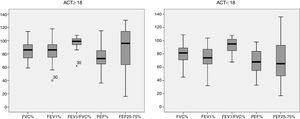
Fig. 1 characterizes the values obtained from the pulmonary function variables of the sample studied, described as a percentage of the predicted value in the literature of each group based on the ACT score. For the group classified as “controlled asthma” (ACT≥18) 56.25% of the patients obtained values above 80% of that predicted in FVC versus 50% of the group classified as “non-controlled asthma” (ACT<18). For FEV1, 53.1% vs. 37.5%; FEV1/FVC 87.5% vs. 72.9%, PEF 34.3% vs. 22.9% and FEF25–75% 56.2% vs. 29.12% respectively.
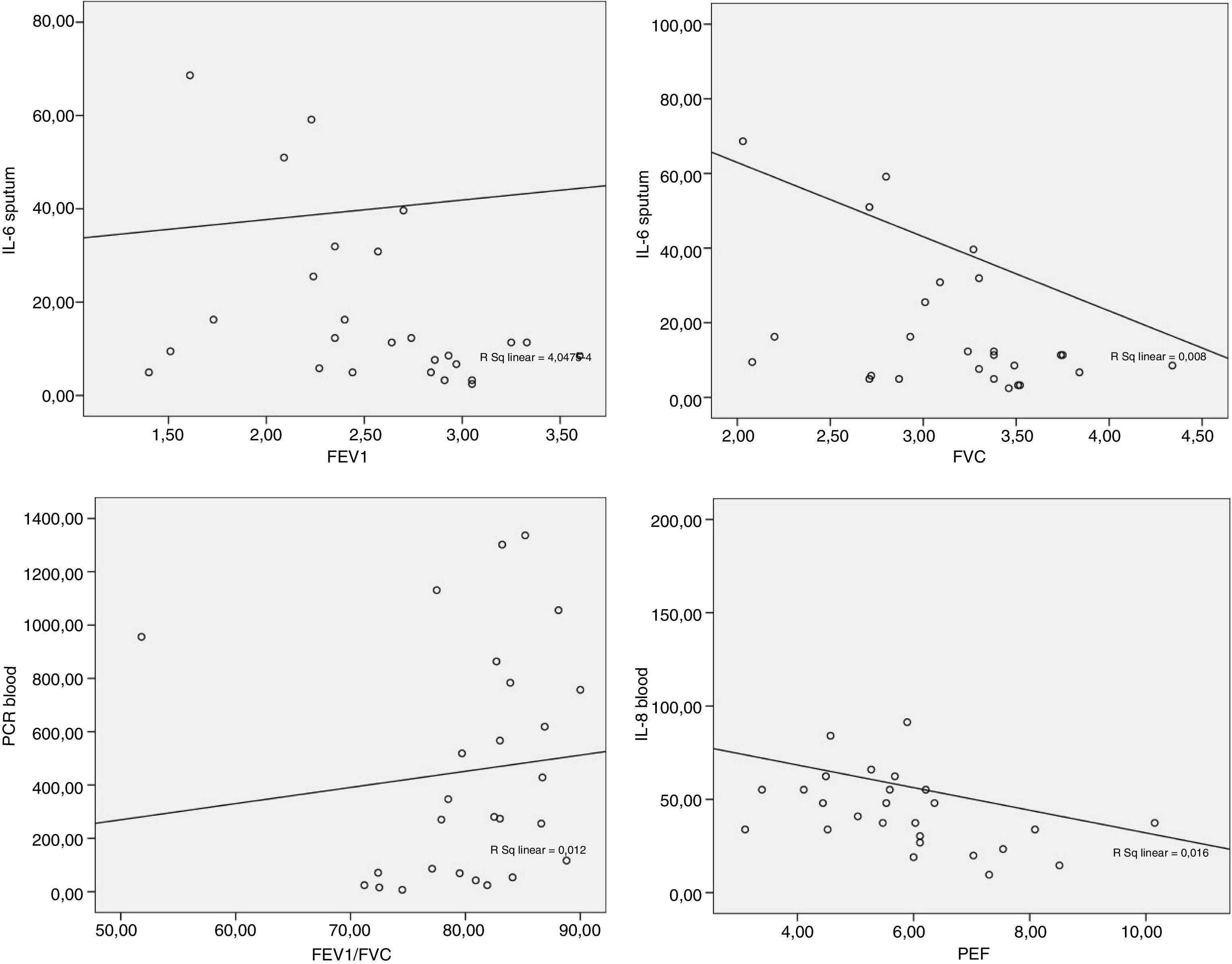
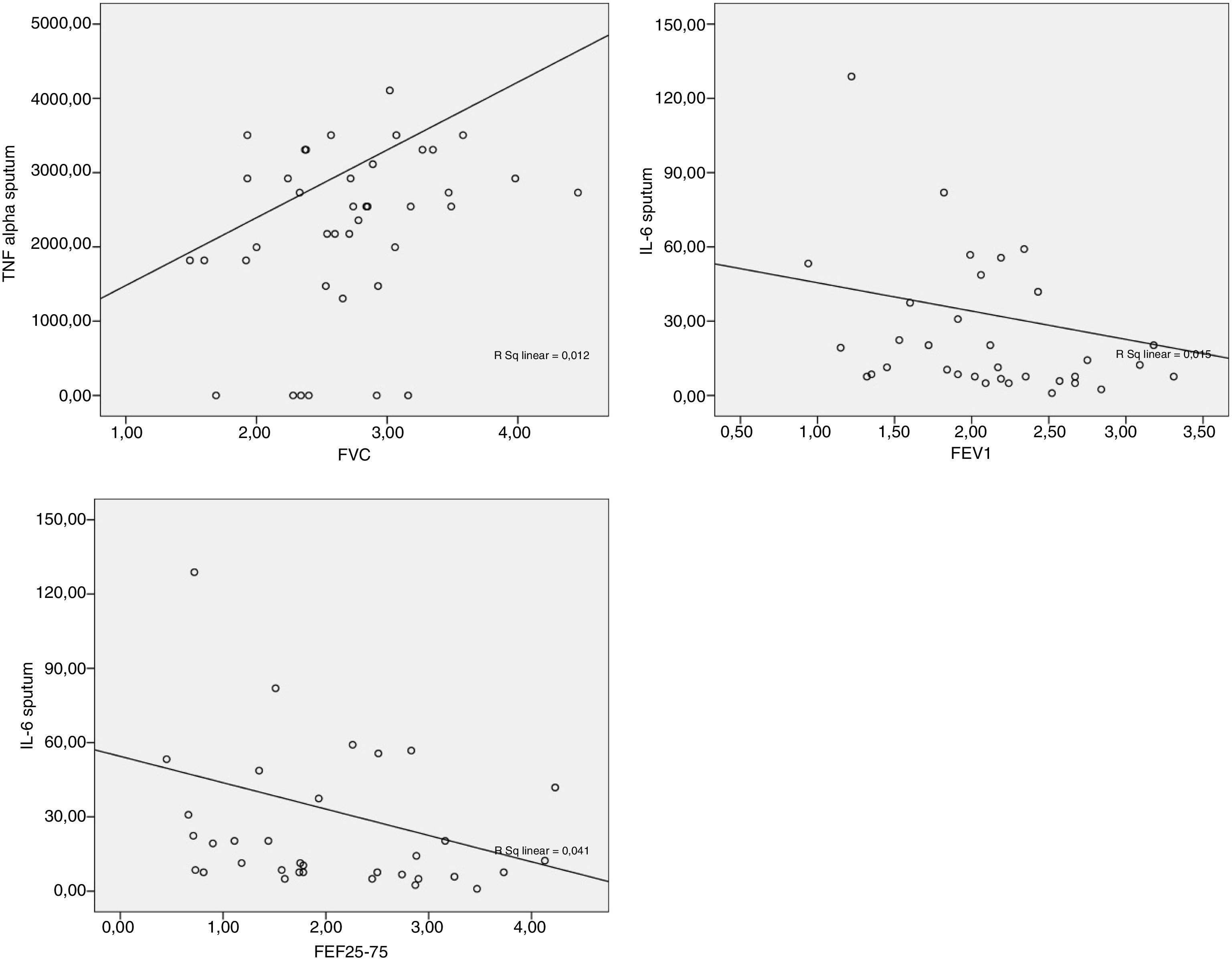
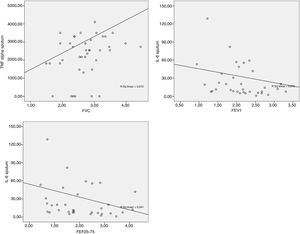
The data obtained from the Spearman correlation between adipokine levels and inflammatory blood and pulmonary sputum mediators with spirometric variables of group classified as “controlled asthma” (ACT≥18) are expressed in Table 2 and Fig. 2. And between adipokine levels and inflammatory blood and pulmonary sputum mediators with spirometric variables of group classified as “non-controlled asthma” (ACT<18) are expressed in Table 3 and Fig. 3.
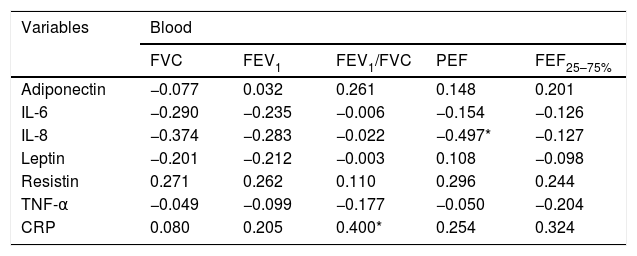
Spearman correlation between adipokine levels and inflammatory blood and pulmonary sputum mediators with spirometric variables of group classified as “controlled asthma” (ACT≥18); values expressed in Spearman correlation coefficient (r).
| Variables | Blood | ||||
|---|---|---|---|---|---|
| FVC | FEV1 | FEV1/FVC | PEF | FEF25–75% | |
| Adiponectin | −0.077 | 0.032 | 0.261 | 0.148 | 0.201 |
| IL-6 | −0.290 | −0.235 | −0.006 | −0.154 | −0.126 |
| IL-8 | −0.374 | −0.283 | −0.022 | −0.497* | −0.127 |
| Leptin | −0.201 | −0.212 | −0.003 | 0.108 | −0.098 |
| Resistin | 0.271 | 0.262 | 0.110 | 0.296 | 0.244 |
| TNF-α | −0.049 | −0.099 | −0.177 | −0.050 | −0.204 |
| CRP | 0.080 | 0.205 | 0.400* | 0.254 | 0.324 |
| Variables | Sputum | ||||
|---|---|---|---|---|---|
| FVC | FEV1 | FEV1/FVC | PEF | FEF25–75% | |
| Adiponectin | −0.026 | −0.038 | −0.057 | 0.123 | −0.033 |
| IL-6 | −0.425* | −0.423* | −0.283 | −0.167 | −0.339 |
| IL-8 | −0.179 | −0.132 | 0.081 | 0.018 | 0.048 |
| Leptin | −0.119 | −0.094 | 0.017 | 0.101 | −0.024 |
| Resistin | −0.097 | −0.078 | −0.005 | 0.225 | 0.007 |
| TNF-α | 0.167 | 0.113 | −0.023 | 0.122 | 0.041 |
| CRP | 0.123 | 0.053 | −0.242 | −0.130 | −0.086 |
FVC: forced vital capacity; FEV1: forced expiratory volume in the 1st second; PEF: peak expiratory flow; FEF25–75%: forced expiratory flow between 25 and 75% of the FVC curve.
*p-value statistically significant with blood CRP×FEV1/FVC p-value=0.039; blood IL-8×PEF p-value=0.008.
**p-value statistically significant with sputum IL-6×FVC p-value=0.027; sputum IL-6×FEV1 p-value=0.028.
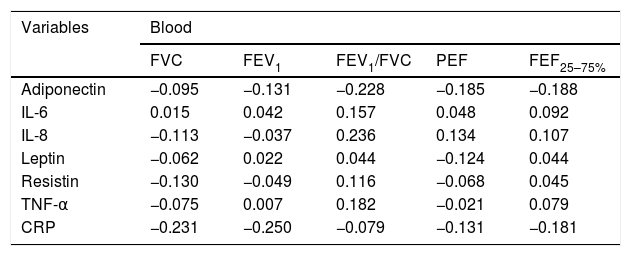
Spearman correlation between adipokine levels and inflammatory blood and pulmonary sputum mediators with spirometric variables of group classified as “non-controlled asthma” (ACT<18); values expressed in Spearman correlation coefficient (r).
| Variables | Blood | ||||
|---|---|---|---|---|---|
| FVC | FEV1 | FEV1/FVC | PEF | FEF25–75% | |
| Adiponectin | −0.095 | −0.131 | −0.228 | −0.185 | −0.188 |
| IL-6 | 0.015 | 0.042 | 0.157 | 0.048 | 0.092 |
| IL-8 | −0.113 | −0.037 | 0.236 | 0.134 | 0.107 |
| Leptin | −0.062 | 0.022 | 0.044 | −0.124 | 0.044 |
| Resistin | −0.130 | −0.049 | 0.116 | −0.068 | 0.045 |
| TNF-α | −0.075 | 0.007 | 0.182 | −0.021 | 0.079 |
| CRP | −0.231 | −0.250 | −0.079 | −0.131 | −0.181 |
| Variables | Sputum | ||||
|---|---|---|---|---|---|
| FVC | FEV1 | FEV1/FVC | PEF | FEF25–75% | |
| Adiponectin | 0.235 | 0.200 | −0.027 | 0.140 | 0.097 |
| IL-6 | −0.227 | −0.358* | −0.323 | −0.247 | −0.354* |
| IL-8 | −0.160 | −0.201 | −0.086 | −0.019 | −0.171 |
| Leptin | −0.096 | −0.078 | −0.096 | 0.003 | −0.067 |
| Resistin | 0.150 | 0.050 | −0.110 | 0.008 | −0.039 |
| TNF-α | 0.315* | 0.205 | −0.086 | 0.038 | 0.043 |
| CRP | 0.107 | 0.088 | 0.042 | −0.029 | 0.038 |
FVC: forced vital capacity; FEV1: forced expiratory volume in the 1st second; PEF: peak expiratory flow; FEF25–75%: forced expiratory flow between 25 and 75% of the FVC curve.
*p-value statistically significant with sputum TNF-α×FVC p-value=0.045; sputum IL-6×FEV1 p-value=0.034; sputum IL-6×FEF25–75% p-value=0.037.
We observed that 60% of the patients did not have controlled asthma in relation to symptoms, medication use and impact on daily activities, directly reflecting on the pulmonary function measurements, with more than half of the patients in this group obtaining measures of FEV1, PEF and FEF25–75% below and 80% of predicted levels. Such measures represents the permeability of small pathways24 and the examination of lung function together with patient's symptomatology and a good clinical evaluation contributes to the pulmonary diseases diagnosis, such as asthma. Epidemiologically, about 300 million individuals are affected by asthma in the world. According to the World Health Organization,25 it is estimated that 235 million people worldwide suffer from asthma. It is a global public health problem, but more than 80% of deaths occur in low and middle-income countries.
The presence of asthma symptoms can cause quality of life impairments, mainly related to environmental stimuli and activity limitation and worse classifications of quality of life is associated with worse FEF25–75%.26
Since adipose tissue is an endocrine organ, the obese individual may present a low-grade systemic inflammatory condition that can affect several organs, including the lungs. Such inflammation may lead to narrowing, airflow obstruction, and premature closure of small airways,3–5,27 causing asthma symptoms.
Bronchial hyperresponsiveness to an inhaled antigen can lead to an inflammatory cascade of airways and degranulation of mast cells, activation of T cells and alveolar macrophages, cytokine production, and recruitment and activation of eosinophils in the airway, resulting in epithelial scaling.28 In view of this, all mechanical and inflammatory changes in the lungs of morbidly obese individuals may lead to changes in the permeability of the smaller airways, reflecting FEV1, PEF and FEF25–75%.13
Thus, the treatment of these patients should involve therapy for weight loss and symptomatic respiratory improvement. There is evidence that weight loss improves levels of inflammatory mediators and adipokines in individuals with grade II and III obesity culminating in better asthma control.9
Most of the studies evaluated by Gupta et al.29 study adipokines, such as adiponectin and leptin, as triggers of the inflammatory alterations found in obese individuals, and the proinflammatory action of leptin is structurally homologous to IL-6 and increases chemotaxis and phagocytosis, leads to cell proliferation T that modulates cytokines and favors the Th1 response rather than Th2. Leptin levels increase in proportion to the increase in BMI.30 On the other hand, adiponectin has anti-inflammatory action and inhibits the production of some inflammatory cytokines such as IL-6 and TNF-α.29
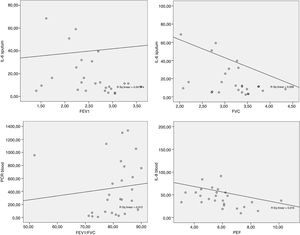
In the present study, there was a significant negative correlation between IL-6 in the sputum with FVC and FEF25-75% in the group ACT<18 and with FVC and FEV1 in the group ACT≥18. The higher the number of pulmonary IL-6, the worse the spirometric values were. The Peters et al.31 study suggests a role for plasma IL-6 in systemic inflammation as a measure of disease severity in an asthmatic patients, as well as an association between increased IL-6 and worse FEV1 in obese individuals.
There is evidence that IL-6 is also related to visceral obesity and adiponectin with subcutaneous obesity in asthmatic women. Visceral obesity is associated with poorly controlled asthma and poor lung function, as well as increased levels of IL-6.32
The present study also found a negative, significant correlation between the blood concentration of IL-8 with PEF in ACT≥18 group. Alveolar macrophages of obese subjects with asthma produce higher amounts of IL-8 and TNF-α after leptin stimulation than macrophages of non-asthmatic obese or eutrophic individuals.33 IL-8 stimulates inflammation by having a pro-inflammatory action and is related to the extent of neutrophilic inflammation due to chemotactic action for neutrophils.34 In addition, obesity alone can promote reduction of lung volumes such as FVC and expiratory reserve volume (EVR)10,35 due to the increase of adipose tissue in the abdominal and thoracic regions causing a restrictive disorder.
Finally, establishing a relationship between the inflammatory aspects of the individual with obesity and their pulmonary function is of great importance in clinical practice in the identification of the symptomatic cause and establishment of effective treatment, such as managing the asthma treatment in the obese in order to improve the profile especially related to interleukin-6 and weight loss as suggested recently.9
ConclusionIt was concluded that the lack of control of asthma symptoms, use of relief medication and interference in daily activities was related to worse lung function, in addition to the increase in pulmonary interleukin-6 being associated with worse lung function even in patients with controlled asthma, especially in the translate airway permeability measures. However, the lack of a control group and the cross-sectional design of this study limit the results extrapolation.