A worldwide rise in weight and obesity is taking place, associated with an increase in several comorbid conditions, such as Obstructive Sleep Apnea (OSA). Bariatric surgery is an effective treatment approach for obesity, with resultant improvement in obesity-related comorbidities. However, the relationship between this type of treatment and OSA is not well established. This systematic review aims to assess and characterize the impact that different types of bariatric surgery have on obese OSA patients. 22 articles with stated preoperative apnea–hypopnea index (AHI), apnea index (AI) or respiratory disturbance index (RDI) were analyzed in this review. A significant improvement in AHI/AI/RDI occurred after surgery, in addition to the foreseeable reduction in body mass index (BMI). Moreover, almost every study stated a postoperative reduction of the AHI to < 20/h and/or a >50% postoperative reduction of AHI, with few exceptions. The interventions with a combined malabsorptive and restrictive mechanism, like roux-en-Y gastric bypass (RYGB), were more efficacious in resolving and improving OSA than purely restrictive ones, like laparoscopic adjustable gastric banding (LAGB).
In conclusion, bariatric surgery has a significant effect on OSA, leading to its resolution or improvement, in the majority of cases, at least in the short/medium term (1–2 years). However, the different results must be interpreted with caution as there are many potential biases resulting from heterogeneous inclusion criteria, duration of follow-up, diagnostic methodology and assessed variables.
Globally, increased weight and obesity are rising in both the developing and developed world.1 Data from the National Health and Nutrition Examination Surveys (NHANES) collected between 2011 and 2012 suggest that 35% of adults are obese,2 and the rates of this disease throughout the world have increased dramatically over the past 40 years and continue to rise in many countries.3 Compared with normal-weight people, obese individuals are responsible for 46% higher inpatient costs, 27% more outpatient visits, and 80% higher spending on prescription medications.2
Obesity is a complex, multifactorial disease that is strongly associated with multiple comorbidities, such as certain types of cancer, cardiovascular disease, disability, diabetes mellitus, gallbladder disease, hypertension, osteoarthritis, sleep apnea and stroke.2
Body mass index (BMI) has been one of the most widely used measures for determining the prevalence of obesity, due to its accuracy in the assessment of body composition, with a high correlation with body fat percentage.4 Obesity is classified as a BMI ≥30kg/m2, with subsequent sub-classification as follows: severe obesity (BMI ≥35kg/m2), morbid obesity (BMI ≥40kg/m2), and super obesity (BMI ≥50kg/m2).4 Bariatric (weight loss) surgery is generally considered for patients with a BMI ≥40kg/m2 or for those with a BMI ≥35kg/m2 with at least 1 serious weight-related comorbid condition, such as type 2 diabetes, Obstructive Sleep Apnea (OSA), or disabling joint disease.3 Laparoscopic gastric banding is also FDA-approved for patients with a BMI ≥30kg/m2 and type 2 diabetes.3 Before having surgery, patients should have made sustained attempts at weight loss with lifestyle modification and/or pharmacotherapy. It is currently standard of care for patients considering bariatric surgery to undergo preoperative psychological evaluation to determine whether surgery is appropriate, and they must also be informed of the potential risks of surgery and the need for long-term monitoring of their weight and nutritional status.3
The diagnosis of OSA is confirmed if the number of obstructive events on a polysomnographic study (apneas, hypopneas+respiratory event related arousals) is greater than 15events/h or greater than 5/h in a patient who reports any of the following: unintentional sleep episodes during wakefulness; daytime sleepiness; unrefreshing sleep; fatigue; insomnia; waking up holding their breath; gasping, or choking; or the bed partner describing loud snoring, breathing interruptions, or both during the patient's sleep.5 The frequency of obstructive events is reported as an apnea–hypopnea index (AHI) or respiratory disturbance index (RDI),5 the main difference being the fact that the latter also takes into account the respiratory event related arousals. OSA severity is defined as mild for AHI or RDI ≥5 and <15, moderate for AHI or RDI ≥15 and ≤30, and severe for AHI or RDI >30/h.5
Obesity is the most significant predisposing factor for OSA.6,7 An elevation of 6kg/m2 in BMI leads to a four times greater risk of developing OSA.7 Central obesity, characterized by fat distribution at the abdominal level, upper body and neck, is the one most associated with OSA.7 Moreover, when compared with normal individuals, obese patients with OSA have 42% more fat in their neck, resulting in pharyngeal lumen narrowing and higher risk of OSA development.8 Another possible pathophysiological mechanism that may explain OSA development in obese patients involves the hormones produced by adipocytes, such as Leptin, whose levels are known to be correlated with human obesity, in a condition known as Leptin resistance.7 This hormone not only has a key role on body-weight control, but also on respiratory center control.9 Thus, increased Leptin levels in a Leptin resistance environment are possibly involved in the pathophysiology of sleep disorders.9,10
In reviewing the literature, this work aims to assess and characterize the impact that different types of bariatric surgery have on obese OSA patients, using AHI/AI(apnea index)/RDI variation.
MethodsA comprehensive search in the “Natural Library of Medicine PUBMed – Medline” was conducted, using the search terms: (bariatric surgery OR obesity surgery) AND sleep apnea, on title or abstract. The inclusion criteria were: studies that specifically assessed the relationship between bariatric surgery and sleep apnea and availability of information regarding AHI/AI/RDI variation with surgery. The exclusion criteria were: studies in a non English language, not conducted in humans and with unstated preoperative AHI/AI/RDI.
Selected articles1032 articles were retrieved from the initial search, of which 989 were rejected after reading the title and abstract, as they met exclusion criteria. From the remaining 43 articles, 21 were also rejected as they did not meet the inclusion criteria. Therefore, 22 articles were analyzed in this review.11–32
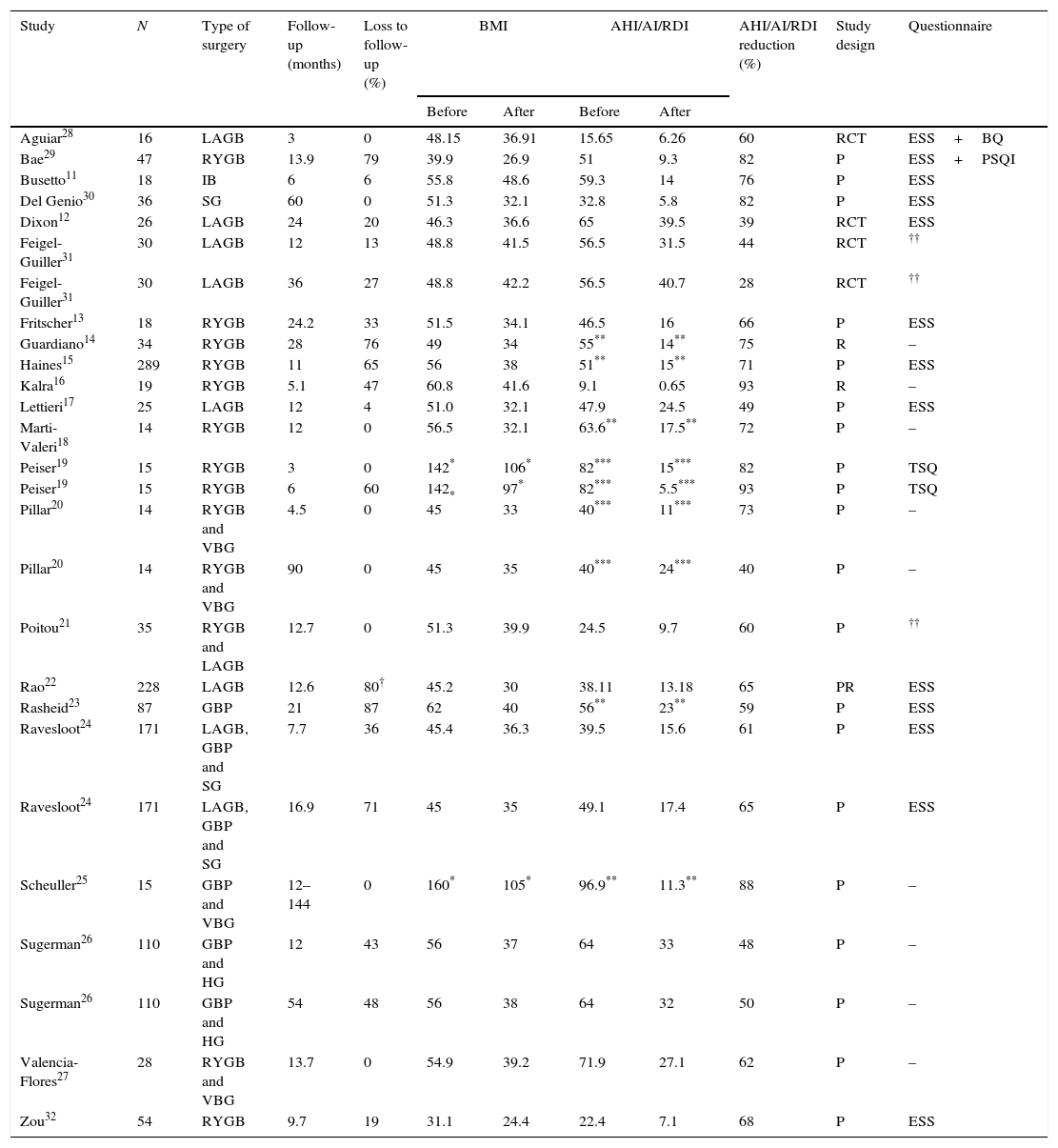
ResultsTable 1 is structured in order to systematically assess the effect of bariatric surgery on the severity of sleep apnea based on the different studies analyzed with stated preoperative AHI/AI/RDI. Moreover, the following information was also collected and organized: time of follow-up; loss to follow-up; BMI and AHI/AI/RDI variation; AHI/AI/RDI reduction after surgery; study design; and type of sleep questionnaire used for symptoms evaluation.
Effect of bariatric surgery on the severity of Obstructive sleep apnea.
| Study | N | Type of surgery | Follow-up (months) | Loss to follow-up (%) | BMI | AHI/AI/RDI | AHI/AI/RDI reduction (%) | Study design | Questionnaire | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||||||
| Aguiar28 | 16 | LAGB | 3 | 0 | 48.15 | 36.91 | 15.65 | 6.26 | 60 | RCT | ESS+BQ |
| Bae29 | 47 | RYGB | 13.9 | 79 | 39.9 | 26.9 | 51 | 9.3 | 82 | P | ESS+PSQI |
| Busetto11 | 18 | IB | 6 | 6 | 55.8 | 48.6 | 59.3 | 14 | 76 | P | ESS |
| Del Genio30 | 36 | SG | 60 | 0 | 51.3 | 32.1 | 32.8 | 5.8 | 82 | P | ESS |
| Dixon12 | 26 | LAGB | 24 | 20 | 46.3 | 36.6 | 65 | 39.5 | 39 | RCT | ESS |
| Feigel-Guiller31 | 30 | LAGB | 12 | 13 | 48.8 | 41.5 | 56.5 | 31.5 | 44 | RCT | †† |
| Feigel-Guiller31 | 30 | LAGB | 36 | 27 | 48.8 | 42.2 | 56.5 | 40.7 | 28 | RCT | †† |
| Fritscher13 | 18 | RYGB | 24.2 | 33 | 51.5 | 34.1 | 46.5 | 16 | 66 | P | ESS |
| Guardiano14 | 34 | RYGB | 28 | 76 | 49 | 34 | 55** | 14** | 75 | R | – |
| Haines15 | 289 | RYGB | 11 | 65 | 56 | 38 | 51** | 15** | 71 | P | ESS |
| Kalra16 | 19 | RYGB | 5.1 | 47 | 60.8 | 41.6 | 9.1 | 0.65 | 93 | R | – |
| Lettieri17 | 25 | LAGB | 12 | 4 | 51.0 | 32.1 | 47.9 | 24.5 | 49 | P | ESS |
| Marti-Valeri18 | 14 | RYGB | 12 | 0 | 56.5 | 32.1 | 63.6** | 17.5** | 72 | P | – |
| Peiser19 | 15 | RYGB | 3 | 0 | 142* | 106* | 82*** | 15*** | 82 | P | TSQ |
| Peiser19 | 15 | RYGB | 6 | 60 | 142* | 97* | 82*** | 5.5*** | 93 | P | TSQ |
| Pillar20 | 14 | RYGB and VBG | 4.5 | 0 | 45 | 33 | 40*** | 11*** | 73 | P | – |
| Pillar20 | 14 | RYGB and VBG | 90 | 0 | 45 | 35 | 40*** | 24*** | 40 | P | – |
| Poitou21 | 35 | RYGB and LAGB | 12.7 | 0 | 51.3 | 39.9 | 24.5 | 9.7 | 60 | P | †† |
| Rao22 | 228 | LAGB | 12.6 | 80† | 45.2 | 30 | 38.11 | 13.18 | 65 | PR | ESS |
| Rasheid23 | 87 | GBP | 21 | 87 | 62 | 40 | 56** | 23** | 59 | P | ESS |
| Ravesloot24 | 171 | LAGB, GBP and SG | 7.7 | 36 | 45.4 | 36.3 | 39.5 | 15.6 | 61 | P | ESS |
| Ravesloot24 | 171 | LAGB, GBP and SG | 16.9 | 71 | 45 | 35 | 49.1 | 17.4 | 65 | P | ESS |
| Scheuller25 | 15 | GBP and VBG | 12–144 | 0 | 160* | 105* | 96.9** | 11.3** | 88 | P | – |
| Sugerman26 | 110 | GBP and HG | 12 | 43 | 56 | 37 | 64 | 33 | 48 | P | – |
| Sugerman26 | 110 | GBP and HG | 54 | 48 | 56 | 38 | 64 | 32 | 50 | P | – |
| Valencia-Flores27 | 28 | RYGB and VBG | 13.7 | 0 | 54.9 | 39.2 | 71.9 | 27.1 | 62 | P | – |
| Zou32 | 54 | RYGB | 9.7 | 19 | 31.1 | 24.4 | 22.4 | 7.1 | 68 | P | ESS |
GBP – gastric bypass; VBG – vertical gastroplasty with band or ring; HG – gastroplasty; IB – intragastric balloon; LAGB – laparoscopic adjustable gastric banding; RYGB – roux-en-Y gastric bypass; SG – sleeve gastrectomy; BMI – body mass index (kg/m2); AHI – apnea–hypopnea index; ESS – Epworth Sleeping Scale; BQ – Berlin Questionnaire; PSQI – Pittsburgh Sleep Quality Index; TSQ – Technion Sleep Questionnaire; P – prospective non randomized (case series); RCT – randomized controlled trial; R – retrospective; PR – prospective randomized.
Used a non specified questionnaire; All studies showed statistically significant associations (p<0.05), except the Scheuller et al.25 study; The majority of studies used full type 1 Polysomnography, except the Busetto et al.11 and the Poitou et al.11 that used cardiorespiratory sleep studies. The Ravesloot et al.11 study used type 1 and type 2 Polysomnography and the Sugerman et al.11 study used type 1 Polysomnography and sleep capneography; N represents the total number of patients with OSA in the beginning of the study, who underwent a bariatric procedure.
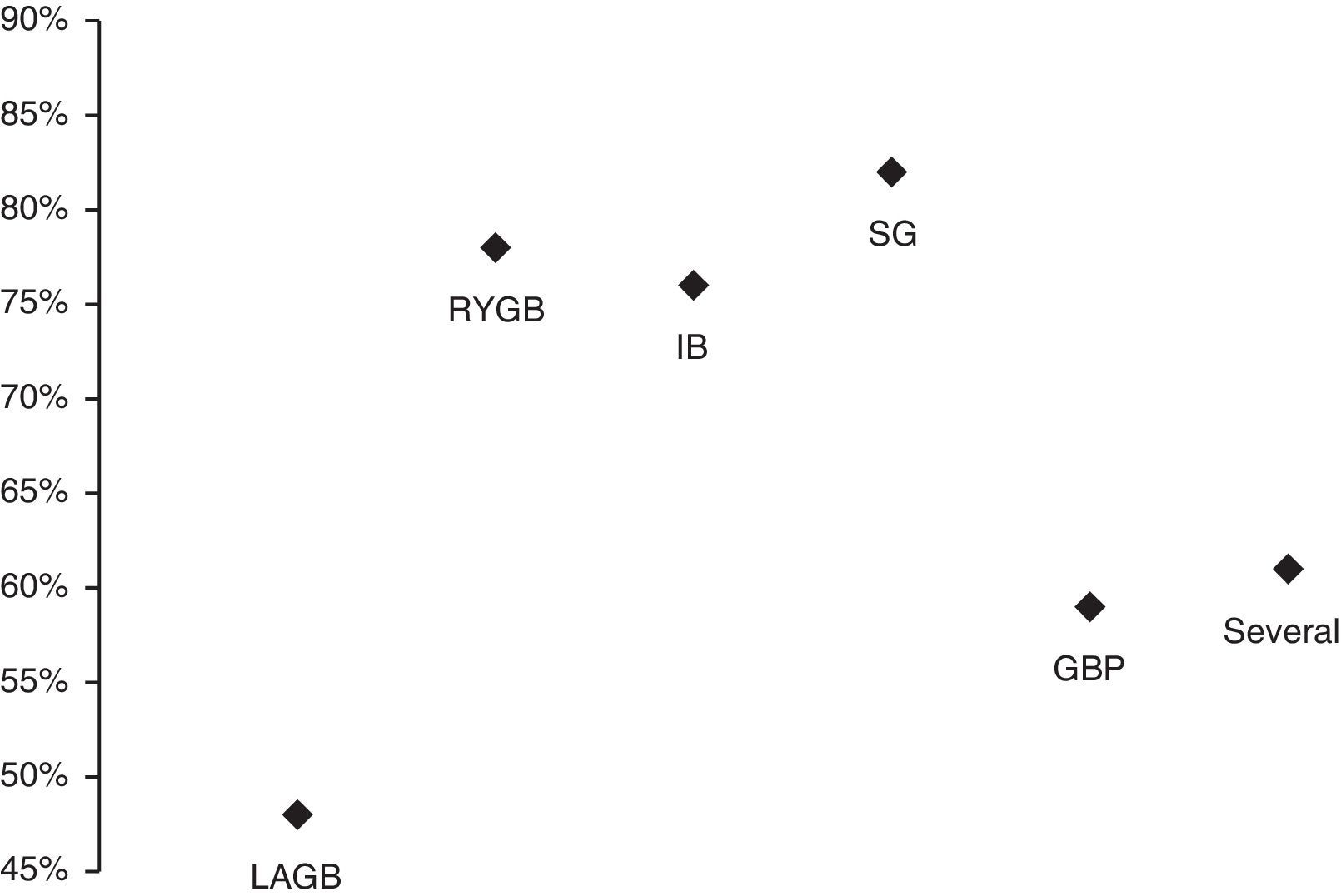
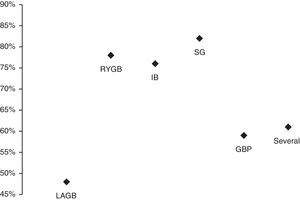
Average AHI/AI/RDI reduction was calculated for each bariatric procedure using the available data from the different studies (Fig. 1).
Percentage of AHI/AI/RDI reduction in each type of bariatric procedure during the following average follow-up periods: IB – intragastric balloon (6 months); Several – more than one procedure (52±39 months); LAGB – laparoscopic adjustable gastric banding (17±13 months); RYGB – roux-en-Y gastric bypass (12±10 months); SG – sleeve gastrectomy (60 months); GBP – gastric bypass (21 months).
As previously stated,3 bariatric surgery not only provides significant, fast and sustained weight loss, as it also provides optimal outcomes in the reduction of frequently associated co-morbidities, such as OSA. The impact of weight loss after bariatric surgery on obese patients with OSA has been assessed through several questionnaires which aim to characterize symptoms and variations in pressure levels used in Continuous Positive Airway Pressure (CPAP), and also OSA severity changes as seen on polysomnography. The success of surgery was defined as a postoperative reduction of the AHI to <20/h and >50% reduction, in patients whose preoperative AHI was >20/h.33 Other authors later proposed tightening these criteria to a postoperative AHI <15 (regarded as “clinically relevant” OSA), <10, and recently even <5.34 One study has considered “response rate” as a reduction of the AHI between 20% and 50%,35 and another study36 has stated that a goal of a mean AHI ≤5, for both surgery and CPAP therapy, is rarely achievable. Therefore, the first mentioned criteria were considered in the present review.
In almost all studies in Table 1, a statistically significant improvement in AHI occurred after surgery, in addition to the foreseeable reduction in BMI, with the exception of the Scheuller et al. study,25 that did not include a ‘p’ value. However, there was a reduction in both AHI and BMI after surgery. Almost every study from this table was conducted in patients with a preoperative AHI >20/h, with the exception of the Aguiar et al.28 and the Kalra et al.16 studies. In the latter, this finding may be explained by the fact that subjects from this study were adolescents with milder forms of the disease. In the former, the fact that the majority of patients were women may justify the lower mean AHI of the group. Almost every study from this table stated a postoperative reduction of the AHI to <20/h, the exceptions being the Dixon et al.,12 Feigel-Guiller et al.,31 Lettieri et al.,17 Pillar et al.,20 Rasheid et al.23 and Sugerman et al.26 studies. The >50% postoperative reduction of AHI was achieved in almost all studies in Table 1, with the exceptions of the Dixon et al.,12 Feigel-Guiller et al.,31 Lettieri et al.,17 Sugerman et al.26 (50% reduction) and Pillar et al.20 studies. In the latter, a significant decrease in AHI from 40/h to 11/h was observed at 4.5 months, followed by a twofold increase to 24/h at 7.5 years, this alteration being independent of BMI variation. Likewise, in the Feigel-Guiller et al.31 study a significant decrease in AHI from 56.5/h to 31.5/h was observed at 12 months, followed by an increase to 40.7/h at 3 years. As a previous systematic review37 stated this may be due to the fact that OSA possibly relapses in the years following surgery, probably attributable to causes other than simply weight gain. Conversely, the longer the period of follow-up, the greater the probability of OSA relapse.37 However, a more recent study38 has shown that these results can be maintained in longer term follow-up. The author concluded that the reduction in intra-abdominal pressure due to excess weight loss following surgery leads to a clinically significant improvement in blood oxygenation, resulting in favorable effects on the cerebral respiratory center.39 Therefore, the results are conflicting but the majority suggests that weight loss persists in short/medium term (1–2 years) and there is a higher probability of OSA relapse after that period. More prospective studies with longer time of follow-up are needed.
The efficacy of the different types of bariatric surgery on improving sleep apnea (AHI/AI/RDI reduction) is represented in Fig. 1. The results obtained with intragastric balloon (IB), sleeve gastrectomy (SG) and gastric bypass (GBP) must be interpreted with caution, as only one study for each one of these techniques was considered. The intervention with a combined malabsorptive and restrictive mechanism, roux-en-Y gastric bypass (RYGB), was more efficacious in improving sleep apnea (higher AHI/AI/RDI reduction) than the purely restrictive one, laparoscopic adjustable gastric banding (LAGB), which simply reduces oral intake. These differences in efficacy have already been described in other studies37,39 and can potentially be explained by the two main factors that contribute to the improvement in OSA following bariatric surgery: weight-dependent effects (decreased mechanical force on the cervical region, upper airway and diaphragm) and weight-independent metabolic effects.39 It is possible to summarize these metabolic effects as the acronym BRAVE: bile flow alteration, restriction of gastric size, anatomical gut rearrangement and altered flow of nutrients, vagal manipulation and enteric gut hormone modulation.39 Another recent study40 stated a correlation between obesity/sleep apnea and systemic inflammation. Furthermore, malabsorptive bariatric techniques reduce several inflammatory biomarkers leading to a protective anti-inflammatory state.40 The most significant and well correlated with sleep apnea biomarker has been shown to be soluble TNF-α receptor 2.40 Moreover, it also seems that a decline in central adiposity and in the production of adipokines that act on the central nervous system, like Leptin, may lead to increased neuromuscular control of pharyngeal diameter.37
Therefore, it makes sense that the studies that did not achieve the considered AHI criteria for a successful surgery (Dixon et al.,12 Feigel-Guiller et al.,31 Lettieri et al.17) used a purely restrictive technique (LAGB).
These reports, however, should be interpreted with caution, as there are some limitations that must be considered. The different time of follow-up between studies is one of the most significant and important issues to take into account. As previously stated, the positive results reported in studies with shorter durations, may be influenced by the simultaneous short-term behavioral changes, such as an increase in exercise and a healthy diet. On the other hand, these same shorter term studies could have had the potential to produce a sustained weight loss and improvement/resolution on OSA (AHI/AI/RDI reduction) that was underestimated. Another important matter is that 15 of the 22 studies that were assessed were from series with loss to follow-up (Table 1) of several patients after surgery, which could have led to biases as the patients with the greatest improvement/results did not drop out, occurring the opposite to the other group of patients. Furthermore, the different patient's preoperative mean BMI and mean AHI considered (Table 1) makes the results very hard to compare and extrapolate between different studies. These values were heterogeneous in the severity of obesity, as well as in the presence and severity of OSA. Moreover, another potential bias arises from the heterogeneous male predominance present in the majority of the analyzed studies, making the extrapolation to the general sleep apnea patients less accurate.
The difference in the study designs used (Table 1) makes the comparison between the analyzed studies more difficult. Moreover, most of them used a full type 1 polysomnography to diagnose sleep apnea but only half of them used the ESS questionnaire to evaluate symptoms, also contributing to a possible bias in the studies comparison.
Finally, generalization of the results to the entire population of patients with OSA and obesity must be done with extreme caution, as in the majority of these studies the leading medical condition was obesity and not sleep apnea. Thereby, the extrapolation to patients whose main medical condition is sleep apnea may be biased.
In summary, bariatric surgery has a significant effect on OSA, leading to resolution or improvement, in the majority of cases, at least in the short/medium term (1–2 years). The combined malabsorptive and restrictive surgical techniques, such as RYGB, seem to be the most efficacious in resolving and improving sleep apnea. The different results must be interpreted with caution as there are many potential biases resulting from heterogeneous inclusion criteria, duration of follow-up, diagnostic methodology and assessed variables. More randomized, controlled trials with homogeneous inclusion criteria, diagnostic methodology and duration of follow-up are needed, in order to assertively confirm the advantageous effects of bariatric surgery on OSA.
Conflicts of interestThe authors have no conflicts of interest to declare.