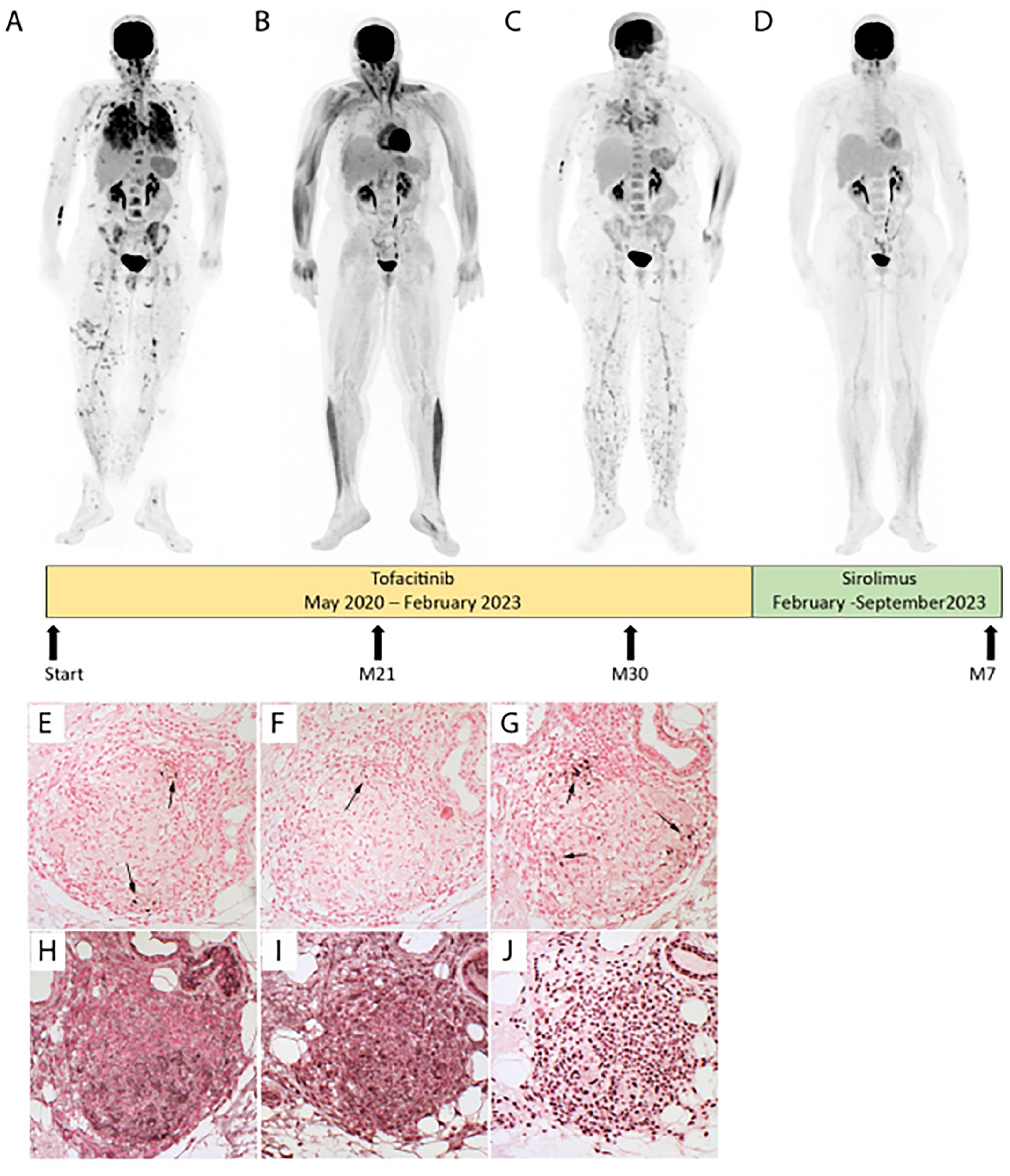
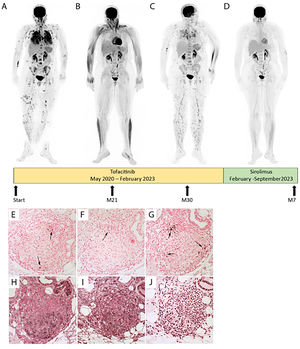
Managing severe pulmonary sarcoidosis is a major clinical challenge, especially when patients fail to respond to or cannot tolerate the first three recommended lines of treatment (steroids, DMARDs and TNFα inhibitors).1 Here, we present a case of severe progressive multivisceral sarcoidosis despite multiple lines of treatment. A 53-years-old Afro-Caribbean woman was diagnosed in 2012 with polyvisceral sarcoidosis. Skin, liver, and bronchial biopsies revealed typical non-caseating granulomas. In 2020, pulmonary infiltration had progressed with significant fibrosis (10%) on thorax computed tomography (CT), accompanied by lupus pernio, hepatic, bone, and splenic involvement. She was treated, on diagnosis, with oral prednisone for two years, rapidly associated with immunosuppressive drugs after weight gain (azathioprine, methotrexate, hydroxychloroquine, infliximab, and adalimumab sequentially). All had to be stopped due to inefficacy or adverse effects. In May 2020, tofacitinib, a JAK inhibitor (JAKi), was initiated (5mg orally every 12h) due to progressive skin lesions, pulmonary worsening on imaging and on pulmonary function (Forced Vital Capacity (FVC) 38% of predicted value (Pred)), emergence of suspected pulmonary hypertension on echocardiography (tricuspid regurgitation velocity at 3.0 m/s), and intense multi-organ disease activity on 18FFDG-PET (Fig. 1A). Tofacitinib led to skin improvement after 3 months, while pulmonary improvement was delayed for 21 months (FVC 50% Pred) with a complete 18FFDG-PET response (Fig. 1B). Her body mass index increased from 24 to 31 kg/m2 after 24 months of tofacitinib, without obvious explanation.
Patient evolution before and after JAKi (Tofacitinib) and mTOR inhibitor (Sirolimus). Evolution of fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) before (A) and after JAKi (Tofacitinib) for 21 months with a complete thoracic, skin, bone, and splenic response (B), or 30 months with a node, skin, lung and splenic relapse (C) or mTOR inhibitor (Sirolimus) for 7 months with a complete multi-organ response (D) after JAKi arrest. P-STAT1 (E), P-STAT3 (F), PSTAT5 (G), P- mTOR (H), P-4E-BP1 (I) and P-P70S6 kinase (J) expression were assayed by immunohistochemistry in serial sections of an archived skin biopsy from the sarcoidosis patient. mTORC1 phosphorylates downstream p70S6 Kinase 1 and modulates the eukaryotic initiation factor 4E-binding protein (4E-BP1), both promoting several cellular processes such as cell proliferation, activation, and survival.
After 30 months, skin lesions and respiratory symptoms resumed (FVC 40% Pred), and 18FFDG-PET revealed multi-organ relapse (Fig. 1C). Sirolimus, a mechanistic target of rapamycin kinase (mTOR) inhibitor, was suggested in a multidisciplinary discussion. Following approval by the French Social Security, sirolimus was initiated in February 2023 at 2 mg once daily, then increased to 4 mg daily to achieve serum concentrations of 5–10 ng/mL. After 7 months of sirolimus, she lost 6 kilograms. Skin lesions and pulmonary condition improved dramatically, with a 10% absolute gain in FVC and a 330-meter improvement on the 6-minute walk test, allowing weaning of supplemental oxygen. At 12 months, FVC reached 54% Pred. Additionally, a complete response was observed on 18FFDG-PET (Fig. 1D).
There is no recommendation regarding fourth-line therapy for refractory pulmonary sarcoidosis, even if several options like JAKi may be considered on a case-by-case basis.1 Several clinical reports and an uncontrolled prospective study, including 10 patients with chronic sarcoidosis treated with JAKi, demonstrated dramatic cutaneous or pulmonary responses.2,3 To the best of our knowledge, JAKi failure either straight away or delayed, as observed in our case report, had never been described in pulmonary sarcoidosis contrary to rheumatoid arthritis, ulcerative colitis, or psoriasis arthritis.
Resistance to JAKi warrants discussion. Medication compliance was certified by interrogating the patient and her pharmacist. She did not drink grapefruit juice nor take any drug-inducer of Cytochrome P3A4, primary metabolizer of tofacitinib. JAKi are not proteins and therefore do not trigger anti-drug antibodies. Increased body weight is not associated with lower plasma drug concentrations, but it may affect inflammatory cytokine levels relevant to sarcoidosis. Indeed, macrophages within dysfunctional adipose tissue acquire pro-inflammatory phenotype, which results in hypersecretion of IL-6 and TNFα.4 In this case, switching to another JAKi or increasing the dose of tofacitinib were excluded due to weight gain, a known adverse effect of tofacitinib. Leptin, a starving hormone, signals through JAK signaling pathway. JAKi could disrupt leptin's ability to signal, leading to abnormal eating and hunger.4 mTOR pathway is implicated in granuloma formation, as supported by animal models, genetic study of familial sarcoidosis, bronchioalveolar lavage transcriptomic analysis, and histologic studies.5 In a prospective single-center study, 7 out 10 patients with glucocorticoid-refractory cutaneous sarcoidosis responded to orally sirolimus during 4 months.6 One case reported a thorax CT response under sirolimus as second line therapy for chronic corticosteroid-dependent non fibrosing pulmonary sarcoidosis.7
An important still unresolved question is how to choose the most appropriate fourth-line treatment, supported by the best rationale, in sarcoidosis. Kraaijvanger et al, showed that JAK/STAT, NLRP3 and mTOR pathways are frequently, but not simultaneously, active.5 Targeted therapies exist for all these pathways and could be the future approach for refractory sarcoidosis treatment. We do not yet have any validated tools to predict response to treatment. Future research should focus on identifying reliable predictors of treatment response; possibly through tissue inflammatory phenotyping, and immunohistochemistry could be an interesting option.5
Interestingly, in this case, immunohistochemical analysis, on a pre tofacitinib skin biopsy, revealed activation of the mTOR signaling pathway (Fig. 1H-J), by contrast to the STAT transcription factors that were barely present (Fig. 1E-G).
In conclusion, this case report illustrates many original points, particularly the evidence of a delayed resistance to JAKi and the remarkable efficacy of sirolimus in a case of severe pulmonary sarcoidosis, not only refractory to glucocorticoids, but also to multiple lines of immunosuppressive therapies.
We would like to thank Gabriel Pop, nuclear medicine physician, for his help in selecting the FDG-PET images and Cécile Rotenberg, pneumologist, for proof reading.