Integrated PET/CT has become a fundamental tool in the preoperative assessment of non-small cell lung cancer (NSCLC) providing useful anatomical and metabolic information to characterize tumoral lesions and to detect unsuspected metastatic disease.
AimTo compare the agreement between clinical and pathological staging before and after the use of PET/CT.
Material and methodsRetrospective study of patients with NSCLC who underwent potentially curative surgery throughout 10.5 years. Cohen's kappa coefficient was used to evaluate staging agreement.
ResultsOne hundred and fifty patients were evaluated, 78% males, with a mean age of 65 (±9.6) years. Thirteen percent were submitted to neoadjuvant chemotherapy. PET/CT was performed in 41%. Global agreement between clinical and pathological staging was 51% (kappa=0.3639). There was a statistically significant difference between the staging results in patients who underwent PET/CT, when compared to the subgroup who did not (p=0.003). For those with PET/CT false negatives occurred in less 39%, false positives in more 12% and clinical and pathological staging coincided in more 27%. The overall results reflected an improvement in the agreement between clinical and pathological staging in the PET/CT subgroup (67%, kappa=0.5737 vs 40%, kappa=0.2292). PET/CT accuracy was enhanced when patients re-staged after neoadjuvant therapy were excluded and a substantial staging agreement was obtained for those who had the exam only for staging purposes (73%, kappa=0.6323).
ConclusionInclusion of PET/CT in NSCLC preoperative assessment improved the accuracy of the clinical staging, with a good level of agreement with pathological staging.
A PET/TC integrada tornou-se num instrumento fundamental na avaliação pré-operatória do cancro do pulmão de não pequenas células (CPNPC), fornecendo informação anatómica e metabólica com utilidade na caracterização das lesões tumorais e na deteção da doença metastática.
ObjetivoComparar a concordância entre o estadiamento clínico e o patológico antes e depois da utilização da PET/TC.
Material e métodosEstudo retrospetivo envolvendo doentes com CPNPC submetidos a cirurgia potencialmente curativa num período de 10,5 anos. O coeficiente de kappa de Cohen foi utilizado para avaliar a concordância entre os resultados.
ResultadosForam incluídos 150 doentes, 78% do sexo masculino, com uma idade média de 65 (± 9,6) anos. Treze por cento foram submetidos a quimioterapia neoadjuvante. A PET/TC foi efetuada em 41%. A concordância global entre o estadiamento clínico e o patológico foi de 51% (kappa = 0,3639). Verificou-se uma diferença estatisticamente significativa quanto aos resultados do estadiamento nos doentes que efetuaram PET/TC em comparação com o subgrupo que não efetuou o exame (p = 0,003). Nos doentes com PET/TC os falsos negativos ocorreram menos 39%, os falsos positivos em mais 12% e o estadiamento clínico e patológico foi coincidente em mais 27% dos casos. Estes resultados refletiram uma melhoria da concordância entre o estadiamento clínico e o patológico no subgrupo com PET/TC (67%, kappa = 0,5737 vs 40%, kappa = 0,2292). A acuidade da PET/TC foi aumentada quando os doentes submetidos a terapêutica neoadjuvante foram excluídos, obtendo-se uma concordância substancial naqueles que efetuaram o exame apenas com o objetivo de estadiamento (73%, kappa = 0,6323).
ConclusãoA inclusão da PET/TC na avaliação pré-operatória do CPNPC melhorou a acuidade do estadiamento clínico, permitindo uma boa concordância com o estadiamento patológico.
Lung cancer remains the most common cause of cancer mortality worldwide, despite advances in diagnosis, staging and therapeutic approaches.1,2
Non-small cell lung cancer (NSCLC) accounts for 75–80% of all cases.3 Since surgery offers the best opportunity for long-term survival and cure,4 appropriate staging is critical in determining treatment options. It is crucial to select suitable patients who have resectable disease and to avoid unnecessary surgery in advanced disease.4,5
NSCLC staging is based on the TNM system, using the tumor (T), node (N) and metastasis (M) evaluations, provided by non-invasive and invasive procedures.4,5
Traditionally, conventional non-invasive procedures include thorax and upper abdomen computed tomography (CT), with some patients requiring specific imaging.4,5 Previous data suggest, however, that staging was not accurate when based on these methods, with a former study demonstrating a slight concordance between clinical and pathological staging (21.7%, kappa=0.0418) in 60 patients with NSCLC submitted to surgery between 1999 and 2003.6
Despite advances in CT scan technology, the morphological information provided has limitations regarding tumor delineation from adjacent structures, limited sensitivity for microscopic disease and is frequently unable to discriminate lymph nodes that are enlarged owing to malignancy or to benign pathology.4,7
The possibility of quantifying the tumor uptake of fluorodeoxyglucose (FDG) and assess its metabolic activity has emerged, in the past two decades, with positron emission tomography (PET). Staging of NSCLC was one of the first approved indications for the use of PET.8 In the meantime, integrated PET/CT, providing both metabolic and anatomic information, has replaced stand-alone PET.9 Integrated PET/CT has the necessary anatomic detail to evaluate the tumor and distinguish malignant lesions from benign lesions with an accuracy of 82%, greater than either CT or PET alone.10 The same is true for lymph nodes evaluation (sensitivity (73%), specificity (80%), positive predictive value (PPV) (78%), negative predictive value (NPV) (91%), accuracy (87%))10 and for malignant extrathoracic metastasis detection (sensitivity (98%), specificity (92%), PPV (89%), NPV (98%)).11 Although PET/CT has demonstrated a good NPV value, it has a lower PPV, indicating that false positives (FP) may occur.10 Tissue sampling, using invasive non-surgical or surgical approaches, is therefore required, in order to confirm positive PET/CT results.
Besides its use for staging purposes, PET/CT also has a role in restaging patients after induction therapy, with literature data showing that it may be better than repeated CT or PET alone,12,13 and even superior to remediastinoscopy.13
PET/CT has become a fundamental technique for the preoperative evaluation of NSCLC and has led to changes in initial staging and treatment plans.14,15
The authors carried out a retrospective study with the main objective of comparing agreement between clinical and pathological staging in patients with NSCLC, operated throughout a period of 10.5 years, including a comparison between two subgroups, for the last of which PET/CT was available.
Materials and methodsA retrospective analysis of clinical files of 150 patients with NSCLC, of the pulmonology department (lung cancer unit) of a university hospital, who underwent surgery over a period of 10.5 years (January 1999–July 2009), was carried out. The authors used data from 60 patients included in a previous study that occurred in the first 5 years of the described period.6
All patients had been recently diagnosed with NSCLC or there were grounds for a very strong suspicion. They were considered operable after physiological assessment and conventional staging procedures with or without whole body integrated PET/CT. Exams were carried out at different institutions, PET/CT was available from 2006. Tumor FDG uptake was qualitative and assessed by comparison to the background activity by visual inspection. Staging was based on the TNM 6th edition.5
All patients underwent thoracotomy and lung resection plus systematic lymphadenectomy. When exploratory thoracotomy revealed an unresectable tumor these patients were excluded.
Results were presented as a percentage for categorical and mean±standard deviation for continuous variables. Cohen's kappa coefficient was used to evaluate the agreement between clinical and pathological staging: 0.0, poor; 0.0–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1, almost perfect.16 Chi square and Student's t-tests were used to compare proportions and continuous variables, respectively. Statistical analysis was done using STATA 9.0 (Statistical/Data Analysis, Stata Corp., College Station, TX 77845, USA). Statistical significance was set to a value of p≤0.05.
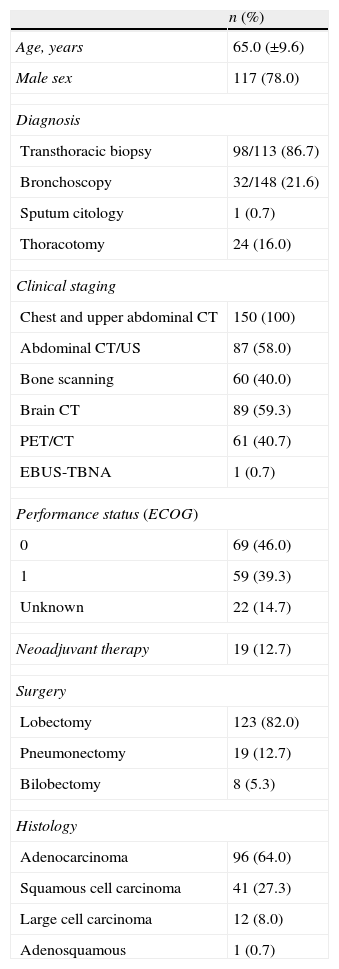
ResultsDemographic and clinical characteristicsThe 150 patients included had a mean age of 65.0 (±9.6) years, the majority were men (n=117, 78.0%) (Table 1). Nineteen (12.7%) underwent neoadjuvant chemotherapy.
Demographic and clinical characteristics (n=150).
| n (%) | |
| Age, years | 65.0 (±9.6) |
| Male sex | 117 (78.0) |
| Diagnosis | |
| Transthoracic biopsy | 98/113 (86.7) |
| Bronchoscopy | 32/148 (21.6) |
| Sputum citology | 1 (0.7) |
| Thoracotomy | 24 (16.0) |
| Clinical staging | |
| Chest and upper abdominal CT | 150 (100) |
| Abdominal CT/US | 87 (58.0) |
| Bone scanning | 60 (40.0) |
| Brain CT | 89 (59.3) |
| PET/CT | 61 (40.7) |
| EBUS-TBNA | 1 (0.7) |
| Performance status (ECOG) | |
| 0 | 69 (46.0) |
| 1 | 59 (39.3) |
| Unknown | 22 (14.7) |
| Neoadjuvant therapy | 19 (12.7) |
| Surgery | |
| Lobectomy | 123 (82.0) |
| Pneumonectomy | 19 (12.7) |
| Bilobectomy | 8 (5.3) |
| Histology | |
| Adenocarcinoma | 96 (64.0) |
| Squamous cell carcinoma | 41 (27.3) |
| Large cell carcinoma | 12 (8.0) |
| Adenosquamous | 1 (0.7) |
Staging PET/CT was performed in 61 (40.7%) patients, in 13 (21.3%) of them this also included restaging. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), available in our setting since 2009, was only used to rule out N2 disease in one patient.
The time between diagnosis and surgery had a mean of 33.7 (±32.9) days for the majority of patients (n=131) and 56.7 (±96.4) for those who had neoadjuvant therapy (n=19). For the neoadjuvant group, there was an average of 30.2 (±15.1) days between restaging PET/CT and surgery.
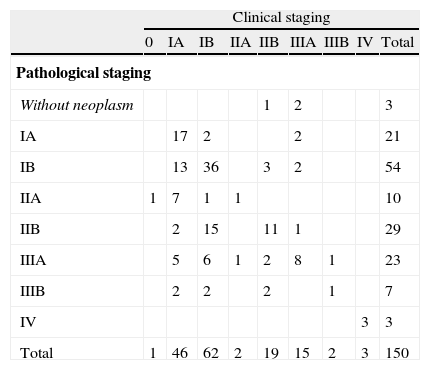
Overall staging resultsMost tumors were clinically classified as being in an early stage: cIB, cIA and cIIB in 62 (41.3%), 46 (30.7%) and 19 (12.7%), respectively (Table 2). Three patients with metastatic disease had previously had surgery for solitary brain metastasis.
Agreement between clinical and pathological stages in the whole population.
| Clinical staging | |||||||||
| 0 | IA | IB | IIA | IIB | IIIA | IIIB | IV | Total | |
| Pathological staging | |||||||||
| Without neoplasm | 1 | 2 | 3 | ||||||
| IA | 17 | 2 | 2 | 21 | |||||
| IB | 13 | 36 | 3 | 2 | 54 | ||||
| IIA | 1 | 7 | 1 | 1 | 10 | ||||
| IIB | 2 | 15 | 11 | 1 | 29 | ||||
| IIIA | 5 | 6 | 1 | 2 | 8 | 1 | 23 | ||
| IIIB | 2 | 2 | 2 | 1 | 7 | ||||
| IV | 3 | 3 | |||||||
| Total | 1 | 46 | 62 | 2 | 19 | 15 | 2 | 3 | 150 |
| Agreement | Expected agreement | Kappa | n |
| 51.33% | 23.50% | 0.3639 | 150 |
Postoperative results demonstrated that in 77 (51.3%) there was a clinical match with pathological staging, in 59 (39.3%) there was upstaging and in 14 (9.3%) downstaging (Table 3). The global agreement was 51.3% (kappa=0.3639). The main changes were: cIB to pIIB (n=15, 20.5%), cIA to pIB (n=13, 17.8%) and cIA to pIIA (n=7, 9.6%).
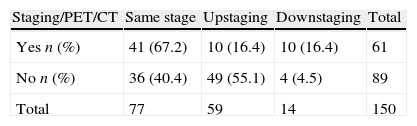
Integration of PET/CT in preoperative evaluationThe authors found a statistically significant difference between clinical and pathological stages in patients who had PET/CT (n=61) as compared to the subgroup who did not (n=89) (p=0.003). For those with PET/CT clinical and pathological staging coincided in more 26.8% (40.4% (n=36) to 67.2% (n=41)), upstaging and false negatives (FN) occurred in less 38.7% (55.1% (n=49) to 16.4% (n=10)) and downstaging and FP in more 11.9% (4.5% (n=4) to 16.4% (n=10)) (Table 3).
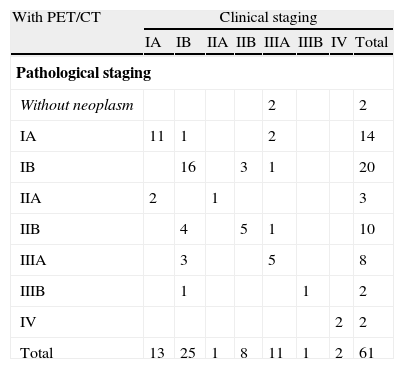
PET/CT false negativesUpstaging occurred in 10 patients (16.9%): cIA (T1N0M0) to pIIA (T1N1M0) in 2; cIB (T2N0M0) to pIIB (T2N1M0) in 4, to pIIIA (T2N2M0) in 3 and to pIIIB (T4N0M0) in 1 (Table 4a).
Agreement between clinical and pathological stages in patients with PET/CT.
| With PET/CT | Clinical staging | |||||||
| IA | IB | IIA | IIB | IIIA | IIIB | IV | Total | |
| Pathological staging | ||||||||
| Without neoplasm | 2 | 2 | ||||||
| IA | 11 | 1 | 2 | 14 | ||||
| IB | 16 | 3 | 1 | 20 | ||||
| IIA | 2 | 1 | 3 | |||||
| IIB | 4 | 5 | 1 | 10 | ||||
| IIIA | 3 | 5 | 8 | |||||
| IIIB | 1 | 1 | 2 | |||||
| IV | 2 | 2 | ||||||
| Total | 13 | 25 | 1 | 8 | 11 | 1 | 2 | 61 |
| Agreement | Expected agreement | Kappa | n | |
| Staging/restaginga | 67.21% | 23.09% | 0.5737 | 61 |
| Staging | 72.92% | 26,35% | 0.6323 | 48 |
In 9 cases (90%) FN occurred due to lymph node underestimation, cN0 to pN1 in 6 patients (66.7%) and cN0 to pN2 in the remaining 3 (33.3%). Among these patients 4 (44.4%) had adenocarcinoma with bronchioloalveolar (BA) component. Tumor size was greater in patients in which cN0 changed to pN2 (45.0 (±2.1) mm) than in those in which it changed to pN1 (23.3 (±0.5) mm), p=0.036. A rise from cT2 to pT4 occurred in one case (10%), also of an adenocarcinoma with mixed pattern, predominantly BA.
PET/CT false positivesA greater variability among tumor and lymph node evaluations was found in the 10 patients (16.9%) in which downstaging occurred: cIB (T2N0M0) to pIA (T1N0M0) in 1; cIIB (T2N1M0 (2) and T3N0M0 (1)) to pIB (T2N0M0) in 3; cIIIA (T2N2M0) to T0N0M0 in 2, to pIA (T1N0M0) in 2, to pIB (T2N0M0) in 1 and to pIIB (T2N1M0) in 1 (Table 4a).
It is worth noting that in none of the 6 patients with cN2 was performed tissue sampling for confirmation of the PET/CT positive result; only one of them had an infection (empyema). In addition, all of the 6 patients (60%) in which we verified a tumor overestimation (4 of them, also with lymph node overestimation) had undergone neoadjuvant therapy and a restaging PET/CT.
Agreement between clinical and pathological staging in PET/CT subgroupThere was an improvement in clinical and pathological staging agreement which resulted in a moderate agreement in patients who underwent PET/CT (67.2%, kappa = 0.5737), when compared to the fair result attained in the subgroup who did not do this exam (40.4%, kappa = 0.2292) (Tables 4a and 4b).
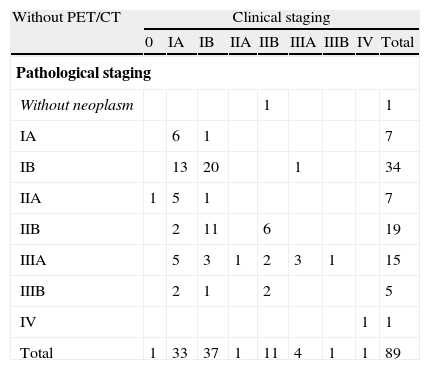
Agreement between clinical and pathological stages in patients without PET/CT.
| Without PET/CT | Clinical staging | ||||||||
| 0 | IA | IB | IIA | IIB | IIIA | IIIB | IV | Total | |
| Pathological staging | |||||||||
| Without neoplasm | 1 | 1 | |||||||
| IA | 6 | 1 | 7 | ||||||
| IB | 13 | 20 | 1 | 34 | |||||
| IIA | 1 | 5 | 1 | 7 | |||||
| IIB | 2 | 11 | 6 | 19 | |||||
| IIIA | 5 | 3 | 1 | 2 | 3 | 1 | 15 | ||
| IIIB | 2 | 1 | 2 | 5 | |||||
| IV | 1 | 1 | |||||||
| Total | 1 | 33 | 37 | 1 | 11 | 4 | 1 | 1 | 89 |
| Agreement | Expected agreement | Kappa | n | |
| Staging/Restaginga | 40.45% | 22.74% | 0.2292 | 89 |
| Staging | 39.76% | 21.37% | 0.2339 | 83 |
PET/CT accuracy was enhanced when patients submitted to neoadjuvant therapy (n=13) were excluded, and a substantial staging agreement was obtained for those who had the exam only for staging purposes (72.9%, kappa = 0.6323) (Table 4a).
DiscussionThis study allowed us to confirm that the inclusion of PET/CT in the preoperative assessment of NSCLC improved clinical staging, giving increased accuracy and the achievement of a good agreement with the pathological staging.
The agreement between clinical and pathological staging in the whole sample of 150 patients was 51.3%. This was a great improvement compared with the agreement obtained in the first study, which was only 21.7% and PET/CT was not available.6 Besides this progress, the overall agreement remains lower than desirable, with upstaging being the main reason for staging modifications.
With PET/CT, upstaging and FN became less frequent than in the subgroup that did not perform this exam (16.4% vs 55.1%) and downstaging and FP more common (16.4% vs 4.5%).
According to the literature FN, in the majority of cases, occurred with small tumors (<7mm in diameter) or tumors with relatively low metabolic activities.17,18 In this study, FN occurred due to a lymph node underestimation in 90.0%, namely cN0 to pN1 in 66.7%, which in practice did not reflect a need for a change in the treatment options. Moreover, adenocarcinoma with BA component was present in 50.0% of all cases, which may reflect the most indolent biological behaviour of this histological subtype.17,18 FP, are mainly documented when there is metabolical activity of infectious or inflammatory lesions.19 The authors found FP occurring after induction chemotherapy and restaging PET/CT in 60.0%. Literature data demonstrated that, although PET/CT has an important role in restaging, its accuracy is lower, and both FP and FN are more frequent.13 Percentage of decrease in the maximum standardized uptake value (SUV) in tumor and lymph nodes may be useful as a predictor of therapy response.12 In addition, the accuracy of PET/CT seems to be higher when it is repeated within 4–12 weeks after induction therapy and close to surgery.12 In our study, FP results may reflect both the unavailability of SUV measuring and the length of time between restaging PET/CT and surgery (30.2 (±15.1) days).
Finally, in 6 patients with PET/CT the positive result in cN2 was not confirmed, which is a limitation to point out. In the meantime, non-surgical approaches, such as EBUS-TBNA and transesophageal endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), became available, although for only one of the included patients. Furthermore, some studies indicate that the combination of EBUS and EUS enable more complete access to the mediastinum, and, in the future, may be considered the gold standard for mediastinal staging.20,21 Additional studies are evaluating the impact of these techniques in the accuracy of staging.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Vaz AP, et al. PET/TC no estadiamento do CPNPC – concordância entre o estadiamento clínico e o patológico. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2012.01.004