Failure of sputum smear and/or culture conversion after 2 months of tuberculosis (TB) treatment has been considered a predictor of patient infectivity and treatment failure. We aimed to identify the factors associated with delayed sputum smear and culture conversion in patients with pulmonary TB who were given anti-TB treatment.
Material and methodsRetrospective cohort of 136 adult patients with sputum culture-proven pulmonary TB referred to an urban Chest Disease Centre. Socio-demographic, clinical, radiological, microbiological, and therapeutic data were evaluated.
ResultsThe median age was 41.0 (interquartile range [IQR] 18.0) years and 75.0% of patients were male. Delayed sputum smear and culture conversion occurred in 25.4% (30/118) and 27.2% (37/136) of patients, respectively. Multivariate analysis indicated that age≥50 years (odds ratio [OR] 4.4, 95% confidence interval [CI] 1.5–13.3), male gender (OR 10.8, 95% CI 1.3–91.1), and smear grade>1–9 acid fast bacilli (AFB)/field (3+) (OR 11.7, 95% CI 1.4–100.6) were significantly associated with persistent smear positivity after 2 months of treatment. Bilateral radiological involvement (OR 3.7, 95% CI 1.5–9.0) and colony count>100 (3+) (OR 5.8, 95% CI 1.2–27.4) were significantly associated with persistent culture positivity.
ConclusionsDelayed sputum smear and culture conversion occurred in about one third of patients. Older age, male gender, and higher bacillary load were independently associated with delayed smear conversion. Bilateral radiological involvement and higher colony count were independently associated with delayed culture conversion.
A ausência de conversão dos exames micobacteriológicos direto e/ou cultural de expetoração após 2 meses de tratamento para a tuberculose (TB) tem sido considerado um preditor do grau de infeciosidade do doente e de falência terapêutica. Os autores estabeleceram por objetivo a identificação dos fatores associados com a conversão tardia dos exames direto e cultural de expetoração num grupo de doentes com TB pulmonar sob tratamento antibacilar.
Material e métodosCoorte retrospetiva de 136 doentes adultos com TB pulmonar, confirmada por exame cultural de expetoração, referenciados a um Centro de Diagnóstico Pneumológico urbano. Foram analisadas variáveis sócio-demográficas, clínicas, radiológicas, microbiológicas e relacionadas com a terapêutica.
ResultadosA mediana de idades foi 41,0 (intervalo interquartil [IIQ] 18,0) anos e 75,0% dos doentes eram do género masculino. A conversão tardia dos exames de expetoração direto e cultural ocorreu em 25,4% (30/118) e 27,2% (37/136) dos doentes, respetivamente. Pela análise multivariada, a idade≥50 anos (odds ratio [OR] 4,4, intervalo de confiança [IC] 95% 1,5–13,3), o género masculino (OR 10,8, IC 95% 1,3–91,1), e a carga bacilar > 1–9 bacilos álcool-ácido resistentes (BAAR)/campo (3+) (OR 11,7, IC 95% 1,4–100,6) estiveram significativamente associados com a positividade persistente do exame direto, após 2 meses de tratamento. O envolvimento radiológico bilateral (OR 3,7, IC 95% 1,5–9,0) e a contagem de colónias>100 (3+) (OR 5,8, IC 95% 1,2–27,4) estiveram significativamente associados com a positividade persistente do exame cultural.
ConclusõesA conversão tardia dos exames direto e cultural de expetoração ocorreu em cerca de um terço dos doentes. A idade mais avançada, o género masculino e a elevada carga bacilar estiveram independentemente associados com a conversão tardia do exame direto de expetoração. O envolvimento radiológico bilateral e a contagem de colónias mais elevada estiveram independentemente associados com a conversão tardia do exame cultural.
Successful control of tuberculosis (TB) depends on early and effective prevention of the transmission of Mycobacterium tuberculosis (MT) from infectious patients. Sputum sterilization is a cardinal index of treatment success and low patient infectivity, and is used to establish the time for airborne isolation in outpatient and inpatient settings. In countries with greater resources, documentation of culture conversion is currently recommended before completion of anti-TB treatment.1,2 Despite the discordance between smear results and culture results (described below), smear microscopy is faster, simpler, and less expensive than culturing, and has thus been widely used for diagnosis and treatment in resource-limited areas.1
Some studies reported that the number of acid-fast bacilli (AFB) decreases rapidly after starting treatment and that 80–85% of TB patients become non-infectious after about 2 weeks.3–5 However, Wang et al.6 estimated that more than 50% of TB patients probably remained infectious after 2 weeks of treatment. This highlights the importance of regular smear and culture monitoring until three consecutive samples are negative in order to establish the time needed for respiratory isolation and to provide proper protection for uninfected contacts. This issue had been previously noted by Clancy.7 Epidemiological studies indicated that the proportion of TB patients who remain smear-positive after 2 months of treatment can be greater than 20% (3.3–25.3%).6–13 Other evidence indicates that failure of smear and/or culture conversion in the second month of TB treatment is a predictor of patient infectivity and treatment failure.6,8–11,14 Thus, identification of the risk factors is very important for TB control policies and for allocation of public health resources. Several studies6,13,15–18 have reported that male gender, diabetes mellitus, smoking, radiologically extensive disease, cavitation, smear grade, and other factors increase the risk for TB infectivity. However, some of the results of these studies were inconsistent due to differences in methodologies, such as the use of smear conversion rather than culture conversion. In particular, previous research has indicated that there can be a discordance between 2-month smear and culture conversion results, and that up to 30% of TB patients who become culture-negative remain smear-positive.19–21 This is due to the presence of nonviable AFB, nontuberculous mycobacteria colonization, or false-positive results.19–21 A recent systematic review and meta-analysis performed by Horne et al. concluded that both smear and culture methods have low sensitivity and specificity for prediction of treatment failure and relapse.22 This position has been argued by some23 in relation to issues concerning methodology, however, Su et al. demonstrated that the limited predictive value of the 2-month smear in culture conversion was due to the significant impact of some clinical factors, beyond smear status, such as cavitation, rifampicin resistance, and use of a directly observed therapy (DOT) strategy.24
The disappearance of AFB from smears and cultures is the most widely accepted determinant for establishing the length of isolation and treatment of patients with pulmonary TB, although caution and clinical judgment must be used in the interpretation of these results, mainly in relation to smear interpretation. The present study was carried out to determine the time to smear and culture conversion and to identify potential predictors for delayed smear and culture conversion in patients with pulmonary TB.
Material and methodsStudy design and populationRetrospective cohort of adult patients with sputum culture-proven pulmonary TB who were referred to an urban Chest Disease Centre (Vila Nova de Gaia, Portugal), from January 2006 to June 2009.
Socio-demographic, clinical, microbiological, radiological, and therapeutic data were collected from patients’ files and the National Plan Against Tuberculosis databases.
The exclusion criteria were: multidrug resistance, inability to collect sputum, default, transfer to another institution, or death during treatment.
Mycobacterial examinations – sputum smear and cultureSputum smears were examined for AFB by fluorescence microscopy and/or Ziehl–Neelsen staining and graded by standard criteria and equivalent: 1–9 AFB/100 fields (1+); 1–9 AFB/10 fields (2+); 1–9 AFB/field (3+); and >9 AFB/field (4+).25,26
Specimens were cultured on the liquid medium BACTEC MGIT 960 (Becton Dickinson) and/or on the Lowenstein–Jensen solid medium (Bio-Rad) and graded as follows: <10 colonies (1+); 10–100 colonies (2+); >100 colonies (3+); and confluence (4+), according to reference laboratory criteria. All first-positive culture examinations were subjected to MT identification tests and drug susceptibility tests (DST) (minimal inhibitory concentration method).
Radiology findingsChest X-rays were characterized according to extension (unilateral, bilateral) and cavitation.
Treatment and monitoringA 6-month standard treatment based on international consensus1 was used (initial phase (2 months): daily isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E); continuance phase (4 months): daily H and R), and changed for patients with concomitant hepatic and/or renal diseases, adverse effects, and DST results. A DOT was given for the entire course of all treatments. Smear and culture examinations were performed at the beginning, at 2 months, and at the end of treatment. However, smears were taken every 2 weeks until there were 3 consecutive negative samples and culture examinations were taken every month until there were 2 consecutive negative samples.27
DefinitionsSmear conversion was defined as 3 consecutive negative samples. Smear and culture conversion time was calculated from the beginning of treatment to the date of the first negative sample. Delayed smear and culture conversion was defined as persistent positivity after 2 months of treatment.
Statistical analysisStatistical analysis was performed using the SPSS version 17.0 software (SPSS Inc., Chicago, Illinois, USA). All probabilities were two-tailed and p values<0.05 were regarded as significant.
Data were described as median and interquartile range (IQR) for quantitative variables (non-normally distributed) and as counts and proportions for qualitative variables. For comparison of quantitative variables the Mann–Whitney test was used. The Chi-square test or the Fisher exact test was used to compare categorical variables whenever was appropriate.
Delayed smear and culture conversion was a dichotomous dependent variable. Variables that were statistically significant and biologically plausible in univariate analysis were entered into a logistic regression model with forward stepwise conditional in order to identify the factors independently associated with that outcome. The odds ratios (OR) and 95% confidence intervals (CI) were determined.
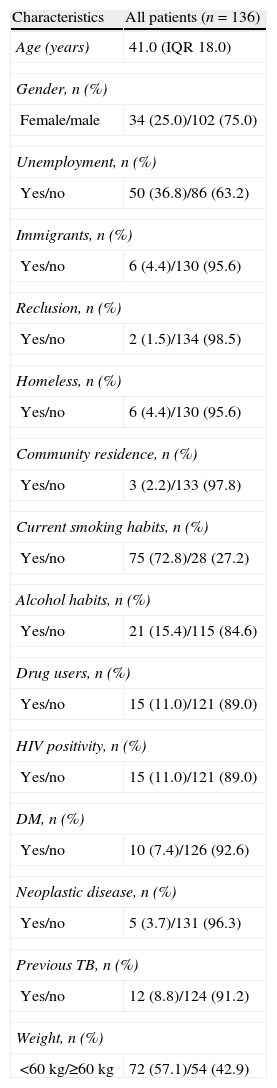
ResultsA total of 136 patients were retrospectively enrolled. The median age was 41.0 (IQR 18.0) years, and 75.0% of the patients were male. Table 1 shows the socio-demographic characteristics and comorbidities of enrolled patients at baseline. All patients had sputum culture-proven pulmonary TB and 118 patients (86.8%) were smear-positive.
Socio-demographic characteristics and comorbidities of baseline population.
| Characteristics | All patients (n=136) |
| Age (years) | 41.0 (IQR 18.0) |
| Gender, n (%) | |
| Female/male | 34 (25.0)/102 (75.0) |
| Unemployment, n (%) | |
| Yes/no | 50 (36.8)/86 (63.2) |
| Immigrants, n (%) | |
| Yes/no | 6 (4.4)/130 (95.6) |
| Reclusion, n (%) | |
| Yes/no | 2 (1.5)/134 (98.5) |
| Homeless, n (%) | |
| Yes/no | 6 (4.4)/130 (95.6) |
| Community residence, n (%) | |
| Yes/no | 3 (2.2)/133 (97.8) |
| Current smoking habits, n (%) | |
| Yes/no | 75 (72.8)/28 (27.2) |
| Alcohol habits, n (%) | |
| Yes/no | 21 (15.4)/115 (84.6) |
| Drug users, n (%) | |
| Yes/no | 15 (11.0)/121 (89.0) |
| HIV positivity, n (%) | |
| Yes/no | 15 (11.0)/121 (89.0) |
| DM, n (%) | |
| Yes/no | 10 (7.4)/126 (92.6) |
| Neoplastic disease, n (%) | |
| Yes/no | 5 (3.7)/131 (96.3) |
| Previous TB, n (%) | |
| Yes/no | 12 (8.8)/124 (91.2) |
| Weight, n (%) | |
| <60kg/≥60kg | 72 (57.1)/54 (42.9) |
Quantitative variables are expressed as median and interquartile range (IQR).
HIV: human immunodeficiency virus; DM: diabetes mellitus; TB: tuberculosis.
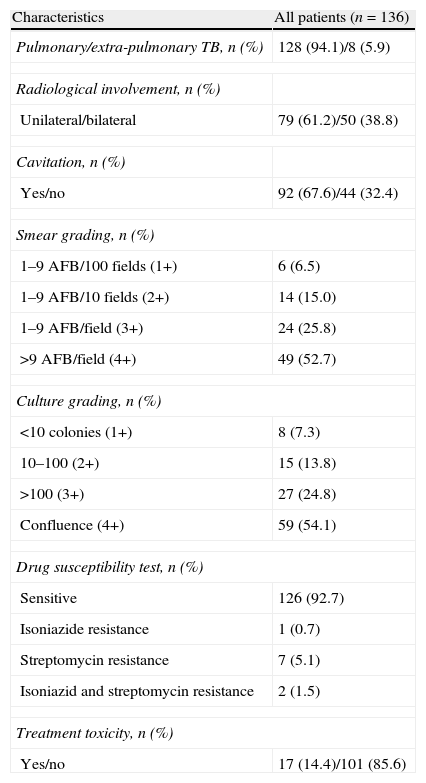
Median smear and culture conversion time was 46 (range 1–343) days and 46 (range 1–127) days, respectively. After 2 months of treatment, 25.4% (30/118) and 27.2% (37/136) of patients remained smear- and culture-positive, respectively. Among the 30 patients with delayed smear conversion, 6 achieved culture conversion. Table 2 shows other characteristics of TB in the enrolled patients.
TB features and treatment.
| Characteristics | All patients (n=136) |
| Pulmonary/extra-pulmonary TB, n (%) | 128 (94.1)/8 (5.9) |
| Radiological involvement, n (%) | |
| Unilateral/bilateral | 79 (61.2)/50 (38.8) |
| Cavitation, n (%) | |
| Yes/no | 92 (67.6)/44 (32.4) |
| Smear grading, n (%) | |
| 1–9 AFB/100 fields (1+) | 6 (6.5) |
| 1–9 AFB/10 fields (2+) | 14 (15.0) |
| 1–9 AFB/field (3+) | 24 (25.8) |
| >9 AFB/field (4+) | 49 (52.7) |
| Culture grading, n (%) | |
| <10 colonies (1+) | 8 (7.3) |
| 10–100 (2+) | 15 (13.8) |
| >100 (3+) | 27 (24.8) |
| Confluence (4+) | 59 (54.1) |
| Drug susceptibility test, n (%) | |
| Sensitive | 126 (92.7) |
| Isoniazide resistance | 1 (0.7) |
| Streptomycin resistance | 7 (5.1) |
| Isoniazid and streptomycin resistance | 2 (1.5) |
| Treatment toxicity, n (%) | |
| Yes/no | 17 (14.4)/101 (85.6) |
TB: tuberculosis; AFB: acid fast bacilli.
Male gender [55.0 (IQR 53.0) days vs 34.0 (IQR 24.5) days; p=0.028], current smoking habits [54.0 (IQR 48.0) days vs 28.0 (IQR 33.5) days; p=0.004], and higher smear gradings [1+: 24.0 (IQR 14.0) days vs 2+: 35.0 (IQR 36.3) days vs 3+: 53.0 (IQR 54.5) days vs 4+: 62.0 (IQR 62.5) days; p=0.001] were associated with longer smear conversion time.
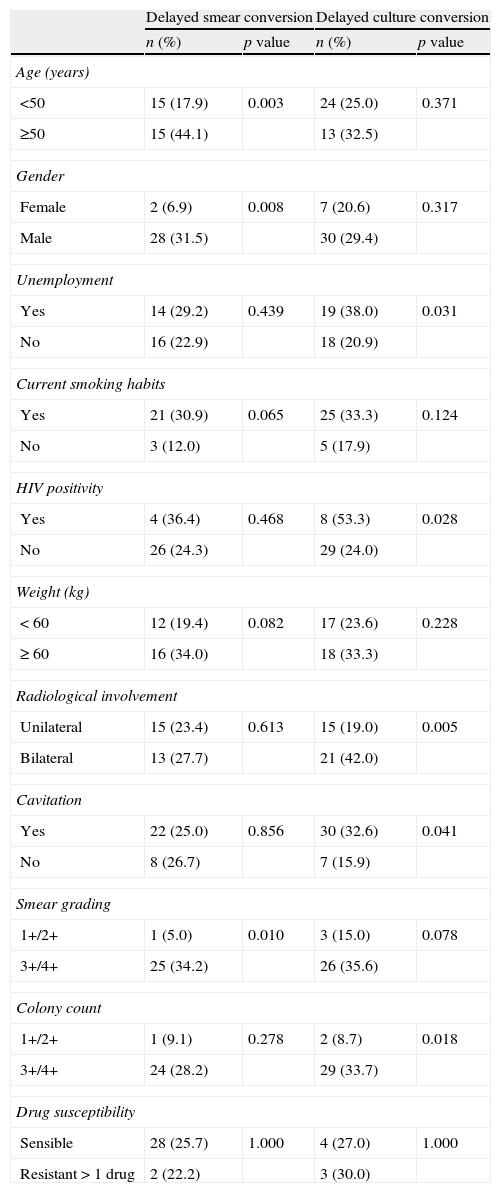
Univariate analysis indicated that the risk of a persistent positive smear at 2 months was greater in patients who were ≥50 years, male, and had a bacillary load >3+ (Table 3). There were no statistically significant differences in other evaluated variables, such as immigrant status, reclusion, homelessness, community residence, alcohol habit, drug abuse, diabetes mellitus, neoplastic disease, previous TB, disease extension (pulmonary or extra-pulmonary), alternative anti-TB treatment, and related toxicity.
Factors related to delayed sputum smear and culture conversiona (univariate analysis).
| Delayed smear conversion | Delayed culture conversion | |||
| n (%) | p value | n (%) | p value | |
| Age (years) | ||||
| <50 | 15 (17.9) | 0.003 | 24 (25.0) | 0.371 |
| ≥50 | 15 (44.1) | 13 (32.5) | ||
| Gender | ||||
| Female | 2 (6.9) | 0.008 | 7 (20.6) | 0.317 |
| Male | 28 (31.5) | 30 (29.4) | ||
| Unemployment | ||||
| Yes | 14 (29.2) | 0.439 | 19 (38.0) | 0.031 |
| No | 16 (22.9) | 18 (20.9) | ||
| Current smoking habits | ||||
| Yes | 21 (30.9) | 0.065 | 25 (33.3) | 0.124 |
| No | 3 (12.0) | 5 (17.9) | ||
| HIV positivity | ||||
| Yes | 4 (36.4) | 0.468 | 8 (53.3) | 0.028 |
| No | 26 (24.3) | 29 (24.0) | ||
| Weight (kg) | ||||
| < 60 | 12 (19.4) | 0.082 | 17 (23.6) | 0.228 |
| ≥ 60 | 16 (34.0) | 18 (33.3) | ||
| Radiological involvement | ||||
| Unilateral | 15 (23.4) | 0.613 | 15 (19.0) | 0.005 |
| Bilateral | 13 (27.7) | 21 (42.0) | ||
| Cavitation | ||||
| Yes | 22 (25.0) | 0.856 | 30 (32.6) | 0.041 |
| No | 8 (26.7) | 7 (15.9) | ||
| Smear grading | ||||
| 1+/2+ | 1 (5.0) | 0.010 | 3 (15.0) | 0.078 |
| 3+/4+ | 25 (34.2) | 26 (35.6) | ||
| Colony count | ||||
| 1+/2+ | 1 (9.1) | 0.278 | 2 (8.7) | 0.018 |
| 3+/4+ | 24 (28.2) | 29 (33.7) | ||
| Drug susceptibility | ||||
| Sensible | 28 (25.7) | 1.000 | 4 (27.0) | 1.000 |
| Resistant>1 drug | 2 (22.2) | 3 (30.0) | ||
HIV: human immunodeficiency virus.
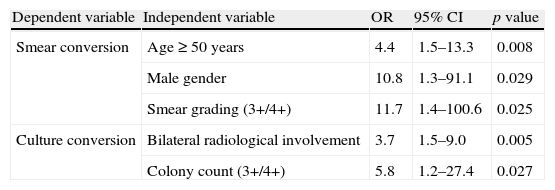
Multivariate logistic regression analysis indicated that all 3 significant variables from the univariate analysis were independently associated with delayed smear conversion (age≥50 years: OR 4.4, 95% CI 1.5–13.3; male gender: OR 10.8, 95% CI 1.3–91.1; bacillary load>3+: OR 11.7, 95% CI 1.4–100.6) (Table 4).
Multivariate logistic regression analysis for variables significantly associated with delayed sputum smear and culture conversion.
| Dependent variable | Independent variable | OR | 95% CI | p value |
| Smear conversion | Age≥50 years | 4.4 | 1.5–13.3 | 0.008 |
| Male gender | 10.8 | 1.3–91.1 | 0.029 | |
| Smear grading (3+/4+) | 11.7 | 1.4–100.6 | 0.025 | |
| Culture conversion | Bilateral radiological involvement | 3.7 | 1.5–9.0 | 0.005 |
| Colony count (3+/4+) | 5.8 | 1.2–27.4 | 0.027 |
Unemployment [56.0 (IQR 55.0) days vs 41.5 (IQR 46.3) days; p=0.046], current smoking habits [58.0 (IQR 47.0) days vs 33.5 (IQR 40.3) days; p=0.022], bilateral radiological involvement [59.5 (IQR 64.8) days vs 41.0 (IQR 43.0) days; p=0.011], cavitary disease [57.0 (IQR 49.8) days vs 35.0 (IQR 42.3) days; p=0.006], and higher colony count [1+: 20.5 (IQR 28.5) days vs 2+: 27.0 (IQR 26.0) days vs 3+: 39.0 (IQR 33.0) days vs 4+: 66.0 (IQR 54.0) days; p<0.001] were associated with longer culture conversion time.
Univariate analysis indicated that the risk of a persistent positive culture at 2 months was greater in patients who were unemployed, HIV-positive, had bilateral radiological involvement, had cavitary disease, and had a colony count>3+ (Table 3).
Multivariate logistic regression analysis indicated that only bilateral radiological involvement (OR 3.7, 95% CI 1.5–9.0) and colony count >3+ (OR 5.8, 95% CI 1.2–27.4) were independently associated with delayed culture conversion (Table 4).
DiscussionRegular sputum smear and culture monitoring during anti-TB treatment allows assessment of sputum conversion, an important issue for therapeutic planning and counseling of TB patients,28 especially in countries such as Portugal29 with an intermediate incidence of TB.
In the present study, we independently analyzed smear and culture results of patients with pulmonary TB. The median times to smear and culture conversion were above one month for both groups, although there were wide and variable ranges which demonstrate the influence of different factors. Current smoking and bacillary load (smear grade and colony count) were associated with long conversion times for both examinations. Male gender was associated with longer smear conversion time and unemployment, bilateral radiological involvement, and cavitary disease were associated with longer culture conversion time. The range of smear conversion time was greater than the range of culture conversion time, presumably due to the presence of nonviable bacilli.20,24
Our results indicated delayed smear and culture conversion in 25.4% and 27.2% of TB patients, respectively. Previous studies have reported varying conversion times in TB patients, and this variation is due to differences in geography, baseline smear status, methodologies, and statistical analysis.6,8–16,18,24,30–32 In the present study, we found that delayed smear conversion was independently associated with age older than 50 years, male gender, and higher pre-treatment smear grade.
Previous studies have shown that lack of smear conversion is more common in older patients,11,15,18,33,34 due to their increased incidence of physical disabilities, the non-efficacious bacilli clearance due to decreased immune response, and delay in seeking diagnosis and care. The potential role of some comorbidities, such as those affecting anti-TB drug absorption and metabolism, may also play a role.
Studies of the effect of gender on sputum conversion are contradictory. In the present study, we found that male gender was associated with delayed smear conversion. Our results agree with those of Rekha et al., who speculated that this was due to the greater prevalence of alcohol consumption and smoking by men.18 In our study, however, we found no effect of gender on alcohol consumption and smoking (data not shown).
We found that patients with high pre-treatment smear grade (3+/4+) were less likely to convert than patients with low pre-treatment grade (1+/2+). Previous studies have reported similar results.6,8,11,35 Rieder reported that smear conversion at 2 months was 90.9%, 77.9%, and 61.7% in patients with initially weak, moderate, and strong positivity, respectively.8 Other studies reported that persistent smear positivity was associated with higher pre-treatment grade due to the initially high mycobacterial burden.36,37
Our univariate analysis indicated that positive culture status at 2 months was associated with unemployment, HIV positivity, bilateral radiological involvement, cavitation, and higher colony count. However, our multivariate analysis indicated that only bilateral radiological involvement and higher colony count were independently associated with that outcome.
Our data indicated that unemployed TB patients had higher prevalence rates of current alcohol consumption, smoking, and HIV positivity (data not shown), so these are potential confounders. Previous evidence has shown that active and passive smokers have an increased risk of contracting active TB compared to non-smokers,38–41 but there is insufficient data on the association between smoking and outcome variables, such as culture conversion.38–41 Our univariate analysis indicated that HIV positivity was associated with delayed culture conversion, but HIV status was not significant in the multivariate analysis. This is in line with some previous studies which have indicated that HIV status does not negatively influence culture conversion, since its positivity has been associated with lower bacillary load expressed, as in our study, by a lower prevalence of cavitary disease in these patients (data not shown).18,30,33,35,42
Our results indicated that bilateral radiological involvement and higher colony count were independent risk factors for delayed culture conversion, due to the high baseline bacillary burden of those patients. In contrast with previous studies,6,11,24,28 our results indicated no relationship of cavitation with delayed smear or culture conversion, although its presence has been significantly associated with a longer time to culture conversion. This discrepancy could be due to differences in methodologies, populations, our small sample size, and/or the retrospective design of our study.
In this study, factors influencing smear and culture conversion were different. Consequently, we can speculate about the importance of evaluating each mycobacterial examination separately. Su et al. performed a prospective analysis of 371 TB patients and reported that the predictive value of the 2-month smear in culture conversion was limited because it was highly influenced by clinical factors, such as initial smear results, cavitation, rifampicin resistance, multidrug resistant TB strains, and DOT.24 Similarly, not all of our patients presented simultaneously with delayed smear and culture conversion.
Knowledge of the risk factors associated with delayed culture conversion assists identification of the highly infectious patients who require the most medical resources and prolonged respiratory isolation, and necessitates a cautious interpretation of sputum smear results. Consequently, some authors22,24 advocate use of a new measure, such as a biomarker with high accuracy and short yield time, rather than culture results, for assessment of treatment response and infectivity during anti-TB treatment.
The main limitations of the present study are the small sample size and its retrospective design, with the consequent data missing and subjective evaluation of some features. The authors tried to minimize these limitations by careful review of all clinical files and available examination results.
In conclusion, our analysis showed delayed smear and culture conversion in about one third of patients. Older age, male gender, and higher bacillary load were independently associated with delayed smear conversion; bilateral radiological involvement and higher colony count were independently associated with delayed culture conversion. We suggest that intensified treatment and precautions against transmission should be especially considered for TB patients with these risk factors, allowing the optimization of national TB control measures.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caetano Mota P. Preditores de conversão tardia dos exames micobacteriológicos directo e cultural de expectoração numa população portuguesa com tuberculose pulmonar. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2011.12.005.