To investigate smoking trajectories and their association with pulmonary function (PF) and respiratory symptoms at age 22.
MethodsData from a population-based cohort study of 3350 individuals and their spirometries were analysed. The outcomes were: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), forced expiratory flow at the mid expiratory phase (FEF25–75 %), FEV1/FVC and FEF25–75/FVC ratio. Smoking data were collected at perinatal follow-up (gestational exposure) and 15, 18 and 22 years. Group-based trajectory model was applied.
ResultsFour groups were identified: no exposure (NE), gestational (GE), gestational and adulthood (GAE) and continuous (CE) exposure. Both CE and GAE trajectories were associated with lower values of FEV1/FVC (−1.77pp; p = 0.01 and −1.58 pp; p<0.001 respectively) and FEF25–75/FVC ratio (−7.27pp; p = 0.019 and −6.04pp; p<0.001 respectively) compared to the NE trajectory. Lower FEV1 and FEF25–75 % values were also related to the GAE trajectory (−68 ml; p = 0.03 and −253 ml/s;p<0.001 respectively). Compared to those who never smoked, individuals who smoked 10 or more cigarettes daily presented a reduction in the FEV1/FVC ratio by 1.37pp (p<0.001), FEF25–75 % by 126 ml (p = 0.012) and FEF25–75 %/FVC ratio by 3.62pp (p = 0.011). CE trajectory showed higher odds of wheezing (OR 4.14; p<0.001) and cough (OR 2.39; p = 0.002) compared to the non-exposed group.
ConclusionsThe in-uterus exposure to maternal smoking reduces PF later in life. However, the perpetuation of smoking behaviour throughout adolescence and early adulthood is determinant for PF main reduction and the emergence of respiratory-related symptoms.
Since the landmark work of Fletcher and Peto,1 insightful updates have been added to the widely accepted causal association between tobacco and lung injury, especially its association with chronic obstructive pulmonary disease (COPD) development risk. Explained for many years by an accelerated lung function decline among smokers, COPD has been recently associated with submaximal levels of pulmonary function (PF) at early adulthood. Since then, a growing body of literature is shifting its attention toward early life disadvantage factors that might be involved in impaired lung growth at the beginning of adulthood, being smoking exposure one of them.2-7
Despite the significant decrease in tobacco use in the last decades in Brazil, due to effective policies in tobacco control,8 smoking at any level remains as one of the major modifiable COPD risk factors, being also markedly related to lung cancer, coronary heart disease, stroke and all-cause mortality.9-12 Over the period from 1990 to 2017, Brazil experienced a decrease in both the prevalence and incidence of chronic respiratory diseases. However, the authors sounded the alarm for the increasing risk of disability-adjusted life years (DALYs) attributed to COPD.8
This study aims to identify different patterns of smoking exposure trajectories and subsequent PF and respiratory-related symptoms at young adult age among participants of the 1993 Pelotas birth cohort, a large ongoing population-based study from Brazil with 22 years of follow-up so far. The dose-response relationship of cigarettes smoked daily with PF at ages 15, 18 and 22 was also addressed. We hypothesize that those who are constantly exposed to tobacco and heavy smokers will present lower PF values at adulthood than those less exposed.
Material and methodsStudy design and sampleThe 1993 Pelotas birth cohort is a prospective study that included neonates who were born and whose mothers resided in the urban area of Pelotas city (southern Brazil) in that calendar year. Of the 5249 individuals from the original cohort, 3350 were included in the present study after providing complete post-bronchodilation PF data at age 22. The details of this cohort study have been previously published.13,14 The Ethics and Research Committee of the Faculty of Medicine of the Federal University of Pelotas (most recent follow-up under the 1.250.366 protocol) approved the study. Written informed consent was obtained from participants or their parents if they were under 18 years old.
Pulmonary function assessmentSpirometry was conducted at age 22 years by a portable ultrasonic spirometer (Easy One model, nDD Medical Technologies Inc., Zurich Switzerland). PF data were accurately assessed by trained technicians, using standard procedures following the American Thoracic Society (ATS) and the European Respiratory Society (ERS recommended guidelines).15,16 A minimum of three and a maximum of eight manoeuvres was performed to obtain at least three acceptable curves, with a maximum difference of 150 ml between the two highest values. Tests were performed before and after administration of salbutamol 400mcg.
The outcomes of interest were: post-bronchodilator values of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) expressed in millilitres (ml); forced expiratory flow at the mid expiratory phase (FEF25–75 %) expressed in millilitres/seconds (ml/s), FEV1/FVC and FEF25–75 %/FVC ratio expressed as percentage points (pp). PF data from 15 to 18 years were also used in supplementary analyses being both measured by the same procedures over the same standardised criteria.
Smoking exposureAt the perinatal follow-up, the assessment of maternal smoking behaviour was recorded by the following question: “Did you smoke during pregnancy?”. At ages 15, 18 and 22, individuals reported their own smoking status. At age 15, current smoking (Yes, No) was defined as ≥1 day of cigarette consumption in the last month, whereas in the remaining ages, smoking status was accessed by the question “Do you smoke?” (at least one cigarette per week in the last month). The number of cigarettes smoked daily in the last month was also addressed at 15, 18 and 22 years of age. Information regarding respiratory symptoms (wheezing, dyspnea, and cough) were accessed at age 22. All information was assessed by ad hoc questionnaires applied by trained interviewers.
Data analysisTo determine the smoking exposure trajectories over time, a group-based trajectory model (GBTM) approach was applied.17,18 Information on maternal smoking status during the gestational period and self-reported smoking at 15, 18 and 22 years were used in the trajectory modelling. Due to binary data (exposed vs. non-exposed), we used the logit-based (LOGIT) model in the TRAJ set of commands in Stata software. After testing different numbers of groups (3, 4 and 5 groups) with different polynomial functions (linear, quadratic or cubic), we selected the best model according to the Bayesian Information Criteria (BIC) and reasonable judgment of other fitting parameters recommended by Nagin18: (a) the average posterior probability – a value above 0.70 is generally recommended; (b) the odds of correct classification: a value above 5.0 shows good assignment accuracy and (c) correspondence between estimated and expected group probabilities – the mismatch between both must be zero for a perfectly fitting model (supplementary table S1).
Description of the sample according to socio-demographic characteristics and smoking exposure was addressed by the Student's t-test and ANOVA. Multivariable linear regression models were used to verify the association between smoking trajectories and PF at age 22. An additional sensitivity analysis verified the effect of the number of cigarettes smoked daily in the last month on PF outcomes. We used a generalized estimating equation (GEE) to obtain the regression coefficients (β). Model selection was performed with the GEE equivalent of the Akaike Information Criterion (AIC), the quasi-likelihood under the independence model criterion (QIC). In this case, instead of trajectories, both the number of cigarettes smoked and PF were measured at 15, 18 and 22 years of age. Individuals were allocated into two groups: one composed of those who smoked 1–9 cigarettes daily and the other one of individuals who smoked 10 or more cigarettes daily. Thus, the change in PF outcomes should be interpreted based on the comparison between each group and the reference (non-smokers).
A logistic regression model analysed the association of smoking trajectories with respiratory-related symptoms such as wheezing, dyspnea and cough at age 22. Heterogeneity p-value between groups was addressed by Chi-squared test across trajectories.
A supplementary analysis was conducted to verify the mean annual change in post-bronchodilator PF according to smoking exposure trajectories by multivariate linear regression. The annual average absolute change on PF parameters was calculated through variance-weighted least square regression (vwls command in Stata) for each individual, considering the parameter and the year of follow-up. This generated a yearly change in the lung function parameter for each individual. Positive values mean an annual increase in the parameter and negative values, the opposite. For example, if an individual has a value of 0.02, it means that the parameter increased 0.02 litres (20 millilitres) per year. We then used this value as the outcome in the model, considering all possible confounders. This model deals with autocorrelation in repeated measures, considering it in the calculation of this annual change.
To assess selection bias, some characteristics of cohort members included (individuals with PF data at age 22) and not included (individuals without PF data at age 22) in the study were compared by Two-sample proportion test.
Covariates used for adjusting means of the outcome were: sex, height (cm), prematurity (gestational age <27 weeks), low birth weight (<2500 g), maternal schooling (years of complete schooling), family income (minimum wages per month in Brazilian currency - a minimum wage equivalent to approximately 84 US dollars), partner and co-worker's smoking exposure during pregnancy collected at the perinatal follow-up, mother and father's smoking exposure at ages 11 and 18 years, household smoking exposure at age 22, family history of asthma and self-reported wheezing and medical diagnosis of asthma collected at 15, 18 and 22 years. All analyses were performed using the Stata Statistical Software (StataCorp LP, College Station, United States), version 16.0. A significance level of 5 % was considered for all tests.
ResultsA total of 3350 individuals from the original cohort who had post-bronchodilator PF measures by the age of 22 years made up the final sample. Compared with those not included in the present analysis (n = 1889), these individuals were more likely to be women and showed higher maternal education and higher family income (supplementary table S2). However, individuals did not differ in terms of smoking exposure.
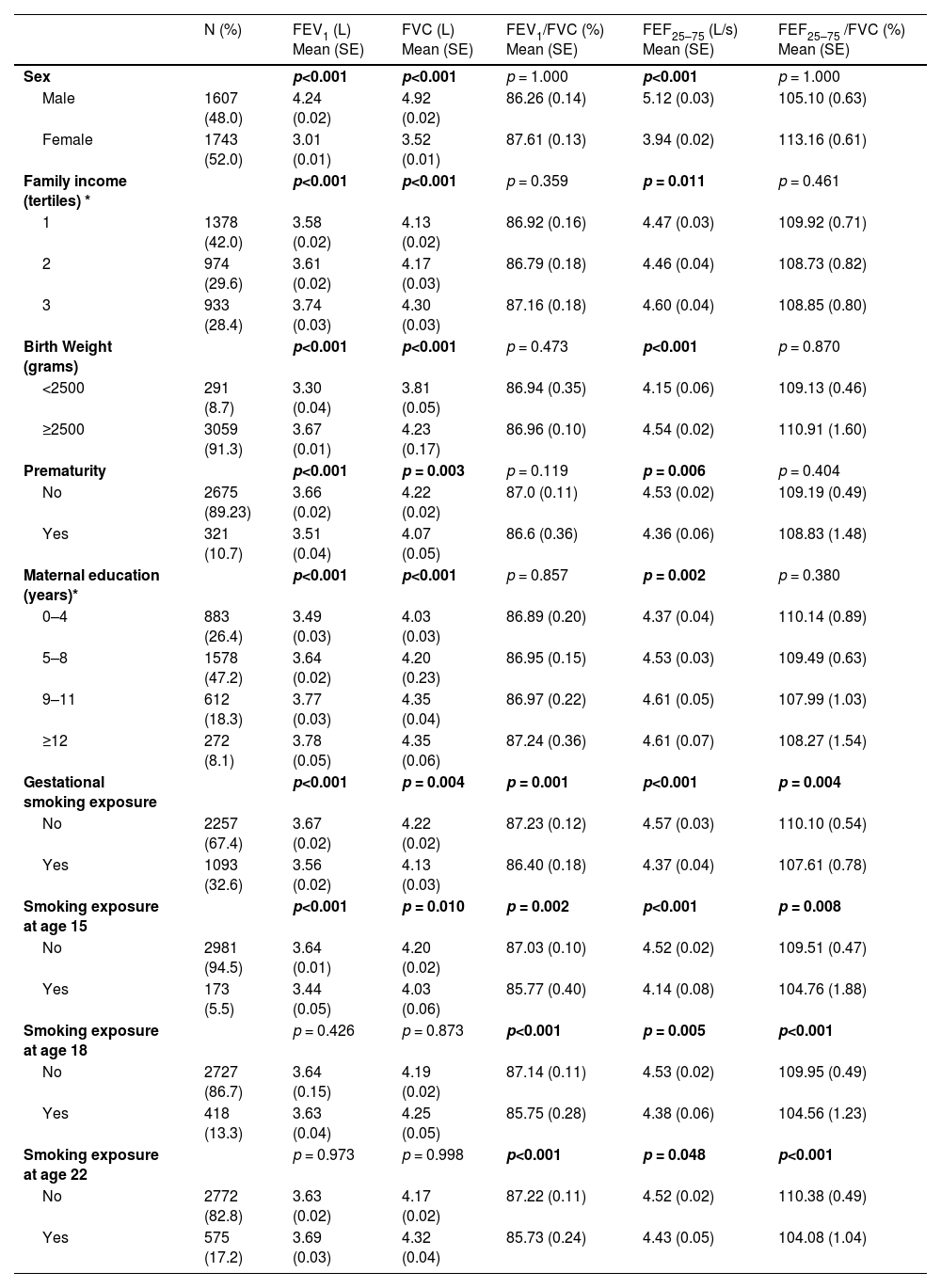
Sample distribution and mean values of PF at age 22 according to socio-demographic characteristics and smoking exposure are shown in Table 1. Approximately half of the study sample were women (52 %), 42 % were born in families belonging to the lowest tertile of income at birth and most of them (73.6 %) had mothers with low education levels (<9 years). At baseline, the prevalence of low birth weight and prematurity among individuals were 8.7 % and 10.7 % respectively. Around 32.6 % of the sample were exposed to tobacco in the uterus, however, the prevalence of non-smokers at the remaining follow-ups were more than 80 %. Lower values of FEV1, FVC and FEF25–75 % were observed in those born prematurely, with low birth weight, belonging to lower-income families and who had lower scholarship mothers (p<0.05). Those who were exposed to tobacco during the gestational period presented lower mean values for all PF parameters (p<0.05). Those who reported being smokers presented lower FEV1/FVC and FEF25–75 %/FVC ratio and FEF25–75 % values compared to non-smokers (p<0.05).
Description of the sample with pulmonary function data at age 22 according to socio-demographic characteristics and smoking exposure (n = 3350).
FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; FEF25–75: forced expiratory flow at the expiratory phase. All information collected at perinatal follow-up, except smoking status at ages 15, 18 and 22 years which were collected at the respective ages. The student's t-test and ANOVA determined p-values. Results are shown as mean and standard error (SE). Bold values indicate p<0.05. *Missing information (maximum of 354 missing for prematurity).
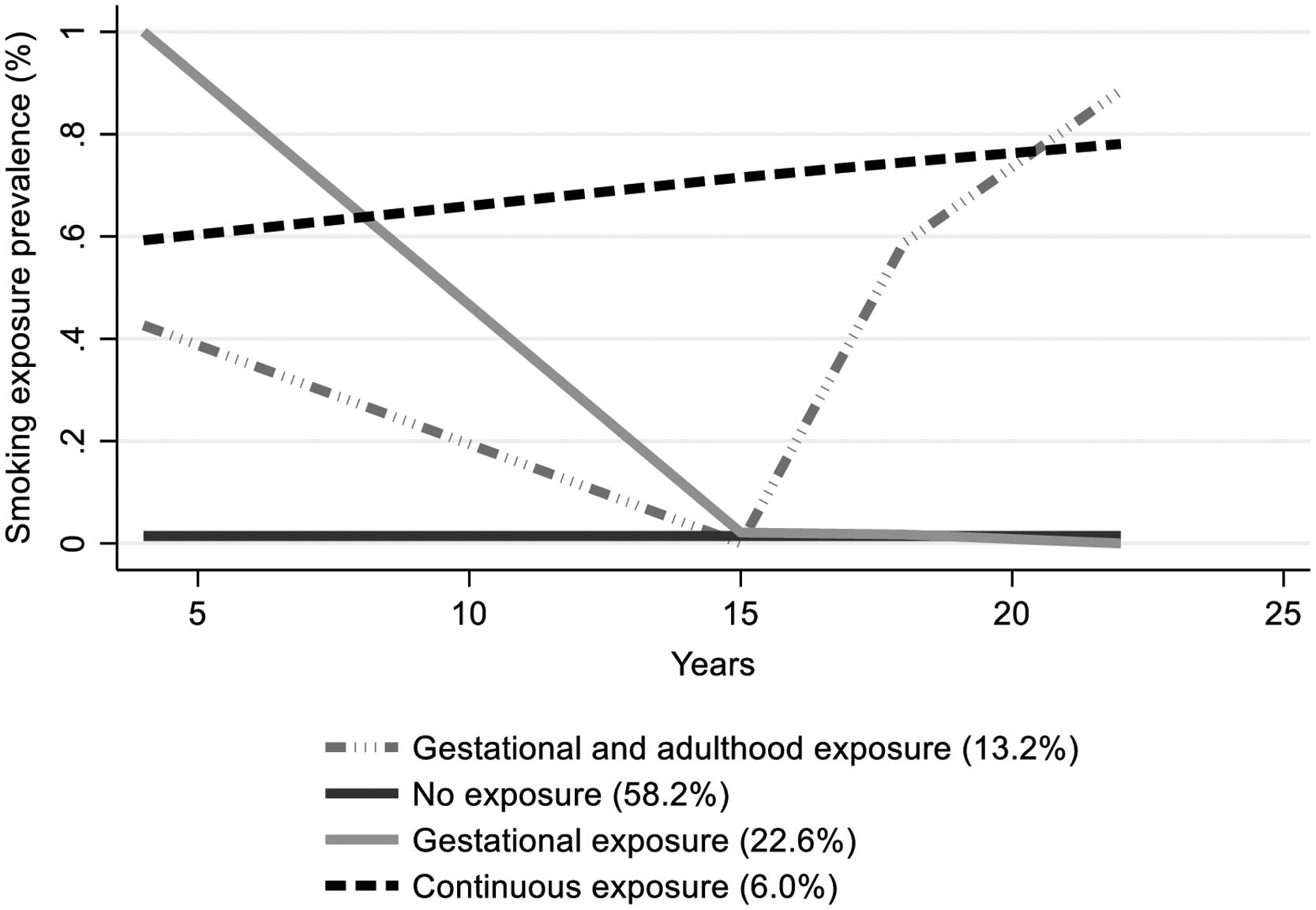
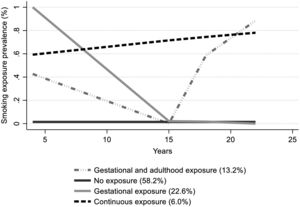
The estimated smoking exposure trajectories are shown in Fig. 1. The best-fitted model (supplementary table S2) presented four trajectories: [1] no exposure (NE); [2] gestational exposure (GE): characterized by the highest smoking prevalence at baseline (around 100 %) and no exposure at adolescence and adulthood; [3] gestational and adulthood exposure (GAE): represented by smoking exposure at gestational period followed by the highest smoking exposure prevalence at adulthood (around 90 %) with no exposure at adolescence; [4] continuous exposure (CE): characterized by a persistent smoking exposure prevalence which slightly increases from 60 % at baseline to over 80 % by the age of 22 years.
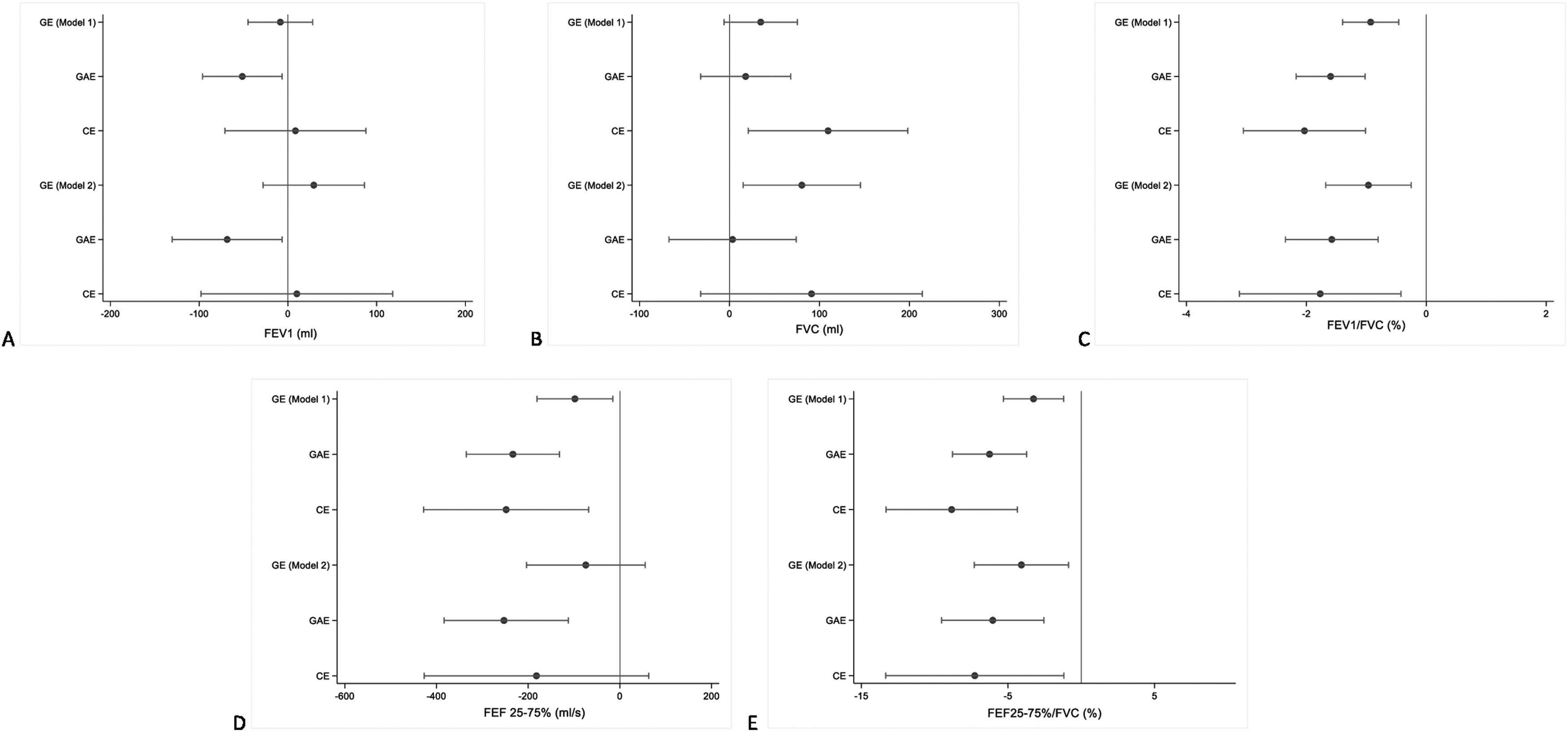
Smoking trajectories were associated with lower PF at age 22 (Fig. 2). When the analysis was adjusted for sex and height (Fig. 2– Model 1), those in the CE and GAE trajectories showed greater PF deficits compared to the NE group (reference). Lower values of FEV1/FVC ratio (−2.03 pp, 95 % CI: −3.05; −1.01 for CE and −1.59 pp, 95 % CI: −2.17; −1.02 for GAE trajectory), FEF25–75 %/FVC ratio (−8.84 pp, 95 % CI: −13.32; −4.36 for CE and −6.26 pp, 95 % CI: −8.79; −3.73 for GAE trajectory) and FEF25–75 % (−248 ml/s, 95 % CI: −428; −68 for CE and −233 ml/s, 95 % CI: −335; −132 for GAE trajectory) were related to both trajectories. After adjustment for a wider set of potential confounders (Fig. 2– model 2), comparing to the NE category, the GAE trajectory was related to lower values of FEV1/FVC ratio (−1.58 pp; 95 % CI: −2.35; −0.80), FEF25–75 %/FVC ratio (−6.04 pp, 95 % CI: −9.53; −2.55) and FEF25–75 % (−253 ml/s; 95 % CI: −393; −112) whereas the CE and the GE trajectories remained significantly related to lower values of both FEV1/FVC ratio (−1.77 pp; 95 % CI: −3.11; −0.42 and −0.96 pp, 95 % CI: −1.68; −0.25 respectively) and FEF25–75 %/FVC ratio (−7.27 pp, 95 % CI: −13.35; −1.18 and −4.08 pp, 95 % CI: −7.30; −0.86 respectively). Lower FEV1 values were only observed in the GAE trajectory (−68 ml; 95 % CI: −130; −6). The GE trajectory was related to a statistically significant increase in FVC (80 ml; 95 % CI: 15; 145).
Mean differences in pulmonary function values measured at age 22 by smoking exposure trajectories. Abbreviations: (A) FEV1: forced expiratory volume in the first second; (B) FVC: forced vital capacity; (C) FEV1/FVC ratio; (D) FEF25–75: forced expiratory flow mid at the expiratory phase; (E) FEF25–75/FVC ratio; GE: gestational exposure; GAE: gestational and adulthood exposure; CE: continuous exposure. Results expressed by regression coefficient (β) and 95 % confidence interval, with no exposure being the reference group. Model 1 adjusted for sex and height. Model 2 adjusted for sex, height, low birth weight, prematurity, family income, maternal schooling, partner and co-worker's smoking exposure during pregnancy, mother and father's smoking exposure at ages 11 and 18 years and household smoking exposure at age 22, family history of asthma, self-reported wheezing and medical diagnosis of asthma collected at 15, 18 and 22 years (n = 3350).
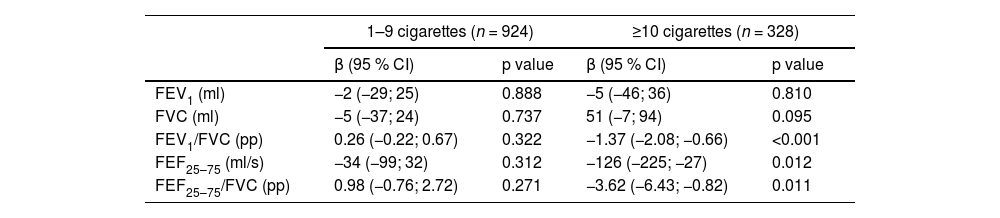
Pulmonary function between ages 15, 18 and 22 was analysed between smoking groups and non-smokers (Table 2). Compared to those who never smoked between ages 15, 18, and 22 years, individuals who smoked 10 or more cigarettes daily presented a reduction in the FEV1/FVC ratio by 1.37 pp (95 % CI: −2.08; −0.66), FEF25–75 % by 126 ml (95 % CI: −225; −27) and the FEF25–75 %/FVC ratio by 3.62 pp (95 % CI: −6.43; −0.82). No difference was found in the PF between those who smoked less than ten cigarettes daily and those who never smoked.
Pulmonary function between ages 15, 18 and 22 years compared by smoking groups and non-smokers.
FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; FEF25–75: forced expiratory flow at the mid expiratory phase. Results are shown as regression coefficient (β) and their respective confidence interval (CI); pp: percentage points. Note: the changes in outcomes are relative to a comparation between smoking groups and non-smokers as the reference group (n = 2324). Model adjusted for sex, height, family income, prematurity, low birth weight, maternal schooling, partner and co-worker's smoking exposure during pregnancy collected at the perinatal follow-up, mother and father's smoking exposure at ages 11 and 18 years and household smoking exposure at age 22, family history of asthma and self-reported wheezing and medical diagnosis of asthma collected at 15, 18 and 22 years.
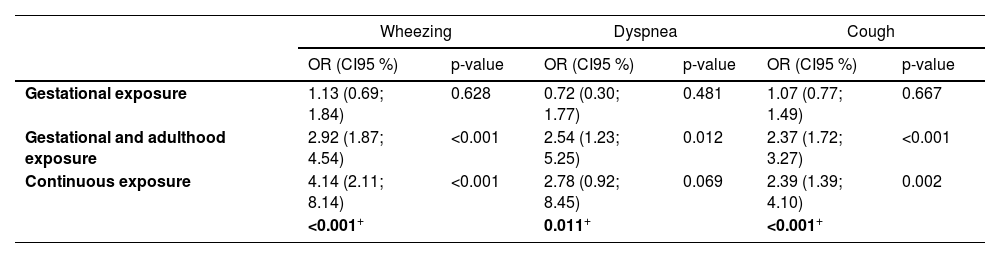
Associations of smoking trajectories with respiratory-related symptoms at age 22 are shown in Table 3. Gestational exposure alone was not associated with later respiratory symptoms. However, individuals belonging to the CE and GAE trajectories presented the higher odds of wheezing (OR 4.14; 95 % CI: 2.11;8.14 and OR 2.92; 95 % CI: 1.87;4.54 respectively) and cough (OR 2.39; 95 % CI: 1.39;4.10 and OR 2.37; 95 % CI: 1.72;3.27 respectively) compared to those who were never exposed. The GAE trajectory also showed higher odds of dyspnea (OR 2.54; 95 % CI: 1.23;5.25). Heterogeneity p-value showed statistical difference between groups estimated effects. The average annual change in PF between 15, 18 and 22 years did not differ between exposed and non-exposed smoking trajectories (supplementary table S3).
Adjusted associations of smoking trajectories with respiratory-related symptoms at age 22: the 1993 Pelotas birth cohort, Brazil (n = 3350).
Logistic regression adjusted to sex, family income, prematurity, low birth weight, maternal schooling, partner and co-worker's smoking exposure during pregnancy collected at the perinatal.
follow-up, mother and father's smoking exposure at ages 11 and 18 years and household smoking exposure at age 22, family history of asthma and self-reported medical diagnosis of asthma collected at 15, 18 and 22 years. Results are shown as Odds Ratio (OR) and their respective confidence interval (CI). + Bold values indicate Heterogeneity p-values addressed by Chi-squared test across trajectories.
By using 22 years of follow-up information, we were able to estimate PF at adulthood within each smoking exposure trajectory. Four trajectories were identified and characterised by different periods and probabilities of exposure throughout the years of follow-up: (NE) zero probability of exposure, which represented the reference group;2 higher probability of exposure during the gestational period only (GE);3 higher probability of exposure at gestational period and adulthood only (GAE);4 probability of exposure in all follow-ups (CE).
The results presented here showed lower PF values among individuals exposed to maternal smoking during pregnancy who also reported themselves as current smokers at adolescence (15 years) and later at ages 18 and 22 years (CE and GAE trajectories).
The GAE trajectory was the only one associated with lower FEV1 values compared to the non-exposed group. Recent findings from the Tasmanian Longitudinal Health Study Cohort (TAHS) showed that an interaction between maternal smoking post-birth and personal smoking enhances the risk of following submaximal FEV1 trajectories until adulthood.7 Similarly, data from the Manchester Asthma Allergy Study Cohort (MASS) and Avon Longitudinal Study of Parents and Children (ALSPAC) revealed that lifelong smoking exposure was also related to lower FEV1 growth.19 However, unlikely our study, smoking exposure was not addressed as trajectories in both studies.
All smoking trajectories were associated with reduced FEV1/FVC ratio when compared to non-smokers. However, only in the GAE trajectory, the lower FEV1/FVC ratio resulted from a significantly reduced FEV1 with no change in FVC. In the remaining smoking trajectories, no differences were observed for FEV1, and the reduced FEV1/FVC ratio was then related to higher values of FVC. In the literature, a reduced FEV1/FVC ratio with normal FEV1 and higher (or normal) FVC has been termed dysanapsis, a mismatch between airway tree calibre and lung size.20 Recent studies significantly related dysanapsis to lower FEV1/FVC ratio (21,22) and to greater COPD risk21 among older adults. These findings provide evidence that even in the absence of a classic pattern of airflow obstruction, i.e. in case of normal FEV1, dysanapsis associated with reduced FEV1/FVC ratio may result in clinical implications to respiratory health.20 Individuals in the GAE trajectory also presented lower FEF25–75 % values, another spirometry measure that comprehends airflow limitation.
Most studies have investigated the effect of either gestational smoking exposure or personal smoking alone on PF growth, but not both. In-utero exposure to tobacco23 and smoking behaviour in adolescence24 were previously related to reduced FEF25–75 % and FEV1/FVC ratio among adolescents. A reduction in FEF25–75 % of −262 ml/s in men aged 21 years had also been associated with in utero tobacco exposure, even after adjusting for birth weight.25 Using path analysis, the Isle of Wight (IOW) cohort found that gestational exposure to maternal smoking was associated directly with a reduction in FEF25–75 % and FEV1/FVC ratio at 18 years.26 Later, assessing individuals PF from age 10 to 26 years, the same cohort demonstrated signs of airflow limitation in current smokers at age 26 (lower FEF25–75 % and FEV1/FVC ratio) compared to non-smokers, with the differences persisting after bronchodilation. No difference was observed for FEV1 though. Smokers also presented a significantly FEF25–75 % decline between ages 18 and 26 years.27
Our findings can be better related to those reported by one study conducted with data from the BAMSE birth cohort (Barn/child, Allergy, Milieu, Stockholm, Epidemiology).28 Although PF was measured at 16 years instead of adulthood and smoking exposure was not explored as a trajectory, the authors also related the combined effects of maternal smoking during the gestational period and adolescent smoking to airway obstruction. Exposure to maternal smoking alone resulted in a reduced FEV1/FVC ratio of −1.1 % (95 % CI −2.0; −0.2), and teenage smoking was associated with a significantly lower FEV1/FVC ratio of −0.9 % (95 % CI −1.8; −0.1 %) compared to the non-exposed group. However, those who were in-utero exposed and smoked at age 16 had a reduced FEV1/FVC ratio of −2.5 % (95 % CI −4.3; −0.7).
Previous studies reported FEF25–75 % as highly sensitive to predicting small airway disease which is affected early in smoke-related lung injuries.27,29 However, a concern relays on the intersubject variability of FEF which originates from its dependency on the FVC. As recommended in the literature, we aimed to correct this drawback by dividing FEF25–75 % to FVC.30 After doing this, individuals belonging to all smoking trajectories showed lower values of FEF25–75 %/FVC ratio compared to those never exposed, which also tends to suggest a small airway disease. This finding can be related to an increased COPD risk in which airway calibre decreases (decreased FEF25–75 %) but the lung volume increases (increased FVC) due to air trapping31 or, as stated previously, due to dysanapsis.21
In our study, FEF25–75 %, FEV1/FVC and FEF25–75 %/FVC ratio were more sensitive than parameters of central obstruction like FEV1 in detecting PF impairment in young adults exposed to tobacco. The FEV1/FVC ratio was decreased by 1.37 pp (95 % CI: −2.08; −0.66), the FEF25–75 % by 126 ml (95 % CI: −225; −27) and the FEF25–75 %/FVC ratio by 3.62 pp (95 % CI: −6.43; −0.82) if ten or more cigarettes were smoked daily compared to never smokers. This finding relates the smoking burden to some extent of airflow limitation in our cohort. An association between the number of cigarettes smoked and PF was previously observed in 10.060 boys and girls aged 10 to 18 years. When compared to never smokers, those belonging to the light (1⁄2 to 4 cigarettes per day), medium (5 to 14 cigarettes per day) and heavy (15 or more cigarettes per day) subgroups of smokers presented lower values of both FEV1/FVC ratio and FEF25–75 %.24
Only individuals who kept smoking throughout adolescence and those who were also currently smokers at young adult age presented respiratory symptoms like wheezing, dyspnea, and cough. These results add some clinical evidence of possible airflow inflammation related to PF loss among those currently exposed to tobacco noxious substances. Our findings may suggest the silent beginning of lung damage developing in those very early exposed, as shown by PF reduction and the appearance of some clinical respiratory symptoms, even if they do not meet the standard threshold of PF impairment that define COPD (such as FEV1/FVC below the lower limit of normal).32 Results from the COPDGene study revealed that accelerated FEV1 decline in COPD, for instance, was preceded by evident signs of radiographically small airways abnormalities at earlier ages, even among individuals without classically defined airflow obstruction.33 This is particularly relevant as the at-risk population shifts younger whereas most COPD studies target individuals with mean ages older than 60 years.34 To effectively reduce the COPD burden related to tobacco exposure, we strongly believe that strategies to promote lung health should include reducing maternal smoking and especially encouraging avoidance of personal smoking.35 Despite PF reduction was related to ten or more cigarettes smoked daily in our study, it does not imply that smoking less is a safe threshold of exposure. Therefore, any amount of cigarette consumption must be avoided.
Strengths of the present study include the evaluation of PF in the emerging adulthood, a crucial period to track individuals who may be at risk of developing many disadvantaged health outcomes related to poor PF.36 The follow-up from birth to young adult age with high participation rates and the use of standardized spirometry tests are additional strengths. A potential limitation of our study is the self-reporting of tobacco exposure, especially regarding maternal smoking status, that may have led to information bias, with underestimation. Self-reported smoking status during pregnancy underestimated the true prevalence of smoker mothers in a previous study.37 Such a misclassification could minimize the effect of smoking patterns on PF. We could not adjust our analyses for second hand smoking exposure nor could we analyse exposure to indoor/outdoor pollution during infancy, which is an important period of lung development. We could not infer a dose response between cigarettes smoked daily and PF as our data were collected in a categorical way. Besides, missing data implied another limitation to our study.
ConclusionsSmoking exposure may compromise lung development leading to reduced maximum attained PF and can also be related with respiratory symptoms like wheezing, dyspnea and cough in early adulthood. Although the exposure to maternal smoking alone also appears to longstanding effect on the lungs, the perpetuation of smoking habit at 15, 18 and 22 years is undoubtedly determinant for lower PF. To guarantee that more individuals reach PF peak values and lifelong respiratory health, smoking cessation policies must be encouraged in all lung growth phases.
CRediT authorship contribution statementP. Weber: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. A.M.B. Menezes: Data curation, Project administration, Writing – review & editing. H. Gonçalves: Data curation, Project administration, Writing – review & editing. P.D. de Oliveira: Data curation, Project administration, Writing – review & editing. A. Wendt: Formal analysis, Writing – review & editing. R. Perez-Padilla: Writing – review & editing. F.C. Wehrmeister: Conceptualization, Data curation, Project administration, Formal analysis, Writing – original draft, Writing – review & editing.
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES). This article is based on data from the study “Pelotas Birth Cohort, 1993” conducted by the Postgraduate Program in Epidemiology of the Federal University of Pelotas with the collaboration of the Brazilian Public Health Association (ABRASCO). From 2004 to 2013, the Wellcome Trust supported the 1993 birth cohort study. The European Union, National Support Program for Centres of Excellence (PRONEX), the Brazilian National Research Council for Scientific and Technological Development (CNPq), and the Brazilian Ministry of Health supported previous phases of the study. The 22-year follow-up was supported by the Science and Technology Department / Brazilian Ministry of Health.
Fernando César Wehrmeister, Ana Maria Baptista Menezes and Helen Gonçalves received scholarship for productivity in research from the Brazilian National Research Council for Scientific and Technological Development (CNPQ) during the conduct of the study. Paula Duarte de Oliveira, Andrea Wendt and Rogelio Perez-Padilla have nothing to disclose.