Pulmonary alveolar proteinosis (PAP) is a rare disorder characterized by abnormal accumulation of a lipoproteinaceous material in the alveoli, which may lead to respiratory failure and has an associated high risk for infections. The mainstay treatment for PAP is whole lung lavage.
A pregnant woman, previously diagnosed with primary PAP, the most common form of PAP, was admitted with dyspnea and worsening respiratory function. In one month period, a wholelung bronchopulmonary lavage was performed twice, with clinical and functional improvement. Pregnancy was carried to term and a healthy baby was delivered.
The mechanisms of respiratory impairment are discussed as well as treatment options and response.
A proteinose alveolar pulmonar (PAP) é uma doença rara caracterizada pela acumulação anormal de material lipoproteináceo nos alvéolos, que pode levar a insuficiência respiratória, estando associada a um risco elevado de infecções. O tratamento gold-standard da PAP é a lavagem pulmonar total.
Uma mulher grávida, com diagnóstico prévio de PAP primária, a forma mais comum de PAP, foi internada com um quadro de dispneia e agravamento da função respiratória. No período de um mês, foi realizada lavagem pulmonar total duas vezes, com melhoria clínica e funcional. A gravidez foi levada a termo, com o nascimento de um bebé saudável.
Os mecanismos de comprometimento respiratório são discutidos, bem como as opções de tratamento e resposta.
PAP is a rare disorder, characterized by abnormal accumulation of a lipoproteinaceous material in alveoli, with minimal interstitial inflammation or fibrosis. There is an increased risk of respiratory infections and clinical course may vary between stable disease with persistent symptoms, spontaneous improvement, and progressive disease evolving to respiratory failure. Whole-lung broncho-pulmonary lavage (BPL) has been the gold standard treatment since the 1960s.
We report a successful full-term pregnancy in a patient with progressive PAP, whose pulmonary function deterioration evolved to such severe respiratory failure, that a whole-lung BPL was necessary. In the literature, a sole case of BPL has been described in a pregnant woman.
Case reportThe patient was a 44-year-old black woman, obese and hypertensive, controlled with nifedipine. She had seven previous pregnancies including one spontaneous abortion and one hydatiform mole. One of her children died from brain cell astrocytoma and the remaining four were healthy.
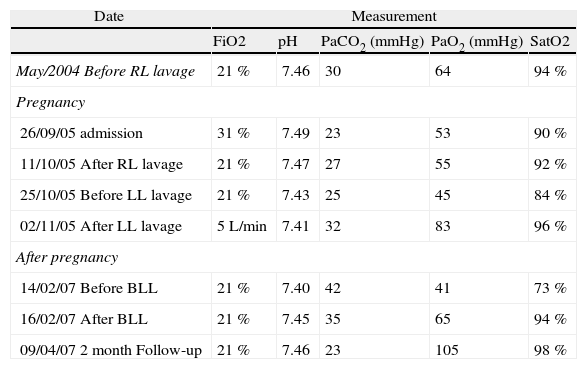
Two years before admission, she had been diagnosed with primary PAP after investigation of complaints of exertional dyspnea, cough and mucous sputum for five months. The diagnosis was based on compatible imaging studies (chest tomography showing interstitial infiltrates and “crazy paving” aspect) and characteristic milky bronchoalveolar-lavage (BAL) fluid with granular material staining with periodic acid-Schiff. Respiratory function test showed a forced expiratory volume in the first second of 1,17L (57 % of predicted), a Tiffeneau index of 65 % and total lung capacity of 3,31L (63 % of predicted). Diffusing capacity (DLCO) was 35 % of predicted. A right whole-lung BPL was performed in May 2004, when symptoms and arterial blood gas (ABG) parameters worsened (Table 1). After this procedure she remained asymptomatic until dyspnea recurred in October 2004, when she refused a new lavage.
Arterial blood gases
| Date | Measurement | ||||
| FiO2 | pH | PaCO2 (mmHg) | PaO2 (mmHg) | SatO2 | |
| May/2004 Before RL lavage | 21 % | 7.46 | 30 | 64 | 94 % |
| Pregnancy | |||||
| 26/09/05 admission | 31 % | 7.49 | 23 | 53 | 90 % |
| 11/10/05 After RL lavage | 21 % | 7.47 | 27 | 55 | 92 % |
| 25/10/05 Before LL lavage | 21 % | 7.43 | 25 | 45 | 84 % |
| 02/11/05 After LL lavage | 5L/min | 7.41 | 32 | 83 | 96 % |
| After pregnancy | |||||
| 14/02/07 Before BLL | 21 % | 7.40 | 42 | 41 | 73 % |
| 16/02/07 After BLL | 21 % | 7.45 | 35 | 65 | 94 % |
| 09/04/07 2 month Follow-up | 21 % | 7.46 | 23 | 105 | 98 % |
BLL: bilateral lung lavage; FiO2: fraction of inspired oxygen; LL: left lung; PaCO2: arterial carbon dioxide tension (mmHg); PaO2: arterial oxygen tension (mmHg); RL: right lung; SatO2: oxygen saturation.
In September 2005, she went to a local hospital with a progressive dyspnea, cough and copious yellow sputum for the previous 4 weeks, fever for 10 days and amenorrhea for 3 months. Antibiotic treatment was unsuccessful, and she was transferred to our hospital due to progressive respiratory failure.
Initial examination revealed obesity (Body mass index of 35), tachypnea, and bibasilar crackles. She had neither ankle edema nor orthopnea. ABG revealed hypoxemic respiratory failure (Table 1). A leucocytosis of 14400/μL and a normal hemoglobin level were present. Chest radiograph illustrated alveolar infiltrates in the inferior thirds bilaterally. Electrocardiogram and saline contrast echocardiogram were normal, excluding right-to-left circulatory shunt; Both BAL fluid (that displayed the characteristic pathologic features of PAP) and blood microbiologic cultures were negative. Although treatment with meropenem resolved leucocytosis, hypoxia persisted and a FiO2 of 40–50 % was necessary to maintain SatO2 above 90 %.
An eight-week pregnancy was disclosed by ultrasound. It was the first pregnancy after the diagnosis of PAP. The risks of life threatening complications were discussed with the patient, who decided to keep the pregnancy and agreed to perform a right whole-lung BPL. The procedure was done in October 6th according to the protocol described by Ben-Abraham in an operating room under general anesthesia and hemodynamic and ventilatory monitoring; almost all of the 9,5L of the instilled fluid was recovered and SatO2 remained always above 90 %. Improvement in respiratory failure was notorious as she decreased the need of oxygen supplements substantially until her release five days later (Table 1).
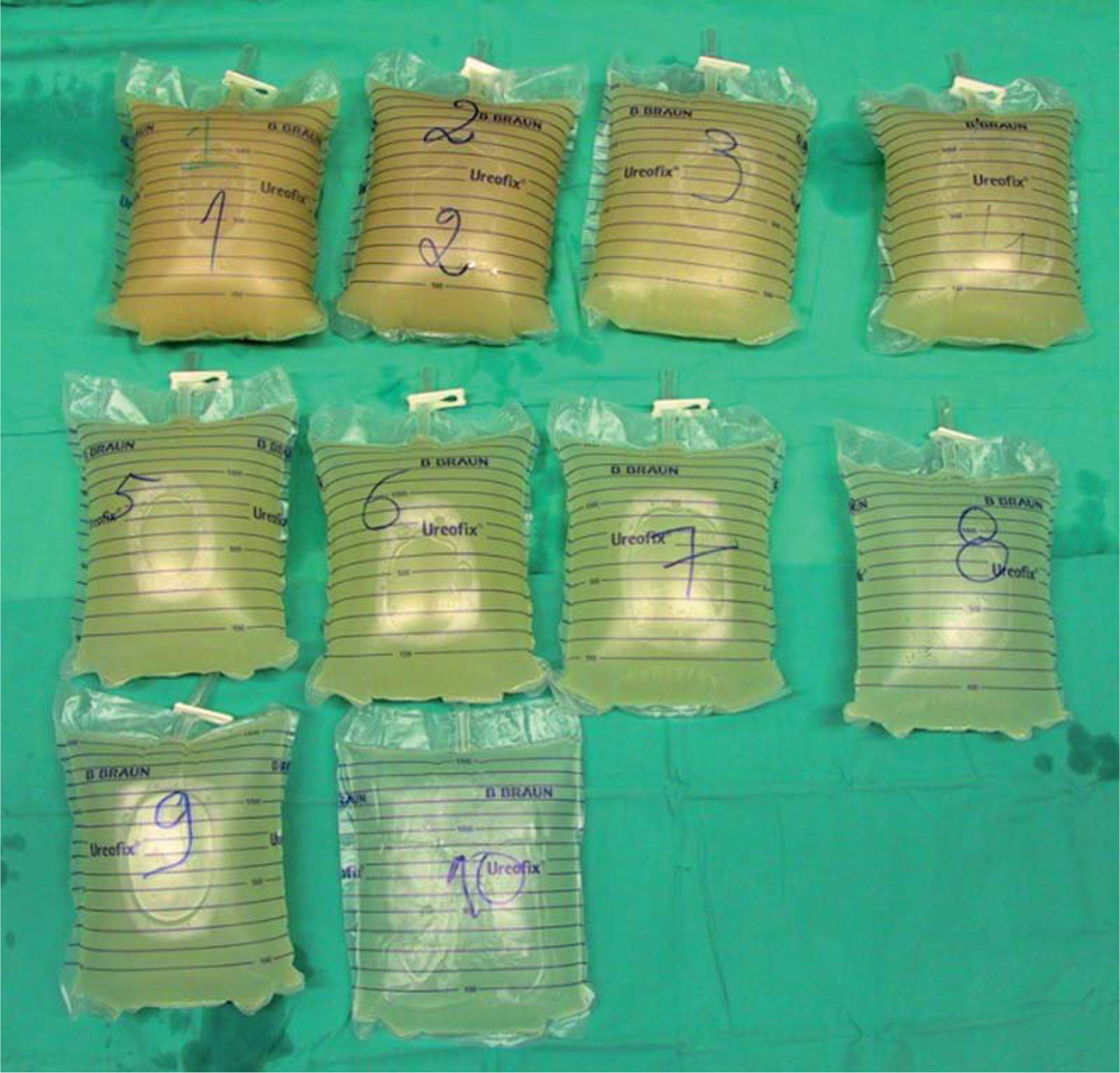
Poor compliance to prescribed oxygen (3L/min) and aggravation of dyspnea were reported at follow-up appointment two weeks later. Gas exchange worsened (Table 1), but there were no signs of infection. She was readmitted for left whole-lung BPL (Fig. 1) with 14L of saline on October 27th (13th week of gestation). After the procedure, although a gas exchange improvement was observed, she remained on oxygen (Table 1). The patient was discharged on oxygen at 4L/min and was closely monitored for the next 13 weeks by Internal medicine and obstetric teams.
On the 26th week of pregnancy, due to progressive dyspnea, the patient was re-admitted and received oxygen through nonrebreathing mask to maintain SatO2 above 93 %. Due to superimposed preeclampsia and breech presentation, a cesarean was performed under epidural anesthesia on the 37th week, and a healthy female baby was delivered. Throughout all the procedures SatO2 remained above 90 %. Postpartum SatO2 was 98 % with a FiO2 of 31 %, and improved in the next six months (SatO2 of 98 % on air).
With time, gas exchange and chest radiography tended to deteriorate (Table 1). On February 2007 a new BPL was performed sequentially in both lungs, resulting in significant improvement in gas exchange and two months later she dropped oxygen supplements (Table 1). Three years later the patient is asymptomatic and with no need of oxygen supplements, while the child exhibits a normal development.
DiscussionThis case of PAP turned out to be very challenging due to the need to manage a pregnant patient with severe hypoxemia in which both treatment and ongoing pregnancy could induce, at least temporarily, a decline in respiratory function. We found a single case of successful whole-lung BPL during pregnancy described in the literature in a young pregnant woman with PAP but healthy otherwise. In our patient, maternal age, hypertension and obesity increased pregnancy risk, making decisions even more difficult.
She presented eight weeks pregnant with respiratory failure and although respiratory infection may have contributed to functional aggravation, it was probably not a preponderant factor, since an improvement was not observed after its resolution. Moreover, later in pregnancy, gas exchange worsening was also observed in the absence of infection. Other causes for the refractory respiratory failure in pregnancy like cardiac failure or right-to-left circulatory shunt were discarded.
Although progression of PAP can not be excluded, the sudden deterioration of pulmonary function coinciding with the beginning of pregnancy and the persistence of respiratory impairment despite pulmonary lavage, suggest that pregnancy had an important role in worsening respiratory failure. Throughout normal pregnancy, anatomical, biochemical and hormonal changes cause adaptations in the respiratory system that compensate for the increased demands of fetal growth and development. These changes are usually well tolerated, however in the setting of underlying pulmonary disease they can lead to deterioration of an already impaired respiratory function. Elevated diaphragms in pregnancy can induce microatelectasis in lower lobes, which could aggravate the ventilation-perfusion mismatch and consequently exacerbate hypoxemia, especially in a patient with ventilatory restriction and severe decrease in DLCO.
Interestingly, PAP has been associated with impaired function of alveolar macrophages (which are responsible for surfactant turnover), caused by auto-antibodies directed at GM-CSF, and is also associated with immunodeficiency states. On the other hand, during pregnancy, besides anatomical and biochemical adaptations, several changes occur in almost every aspect of the immune system. For instance, these changes are believed to modify the course of auto-immune diseases due to alterations in the activity of some immunity cells. Hence it is possible to reason that the known immunomodulation occurring in pregnancy could contribute to impaired macrophage function (with additional imbalance in surfactant homeostasis), thereby worsening clinical condition. This could explain ventilatory decline at an early stage of pregnancy, when the influence of the uterus growth is not yet noticed. Further studies are required to clarify this question.
BPL, the gold-standard treatment for PAP, is described as safe and efficient, resulting in increased survival. It can be performed one lung at a time or in both lungs sequentially if patient is considered to tolerate one-lung ventilation of the lung that has just been treated.10,11 Improvement in arterial oxygen tension can be observed after one week. In this case, dyspnea, impact on daily activities and development of respiratory failure were indications to BPL10,12 and actual improvement was noted after the procedures, allowing significant decrease in oxygen needs.
In conclusion, several aspects may have concurred to the severe hypoxemia observed, including PAP, obesity and anatomophysiologic changes of pregnancy. This case demonstrates that it is possible to carry out a full-term pregnancy in a patient with a serious pulmonary disease as PAP, as long as close monitoring of mother and fetus, as well as specific treatments (BPL included) and supplemental oxygenation are provided.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access