Tumor necrosis factor-alpha (TNF-α) is a pleomorphic cytokine involved in the pathogenesis of many rheumatic diseases. Following anti-TNF therapy, the relative risk for tuberculosis is increased by up to 25 times, often as a rapidly progressive disease, extra-pulmonary or disseminated.1–4 Aiming to evaluate compliance with guidelines for tuberculosis screening, the authors conducted an enquiry among northern Portuguese hospitals which prescribe anti-TNF therapy.
From all hospitals in northern Portugal the authors identified those that prescribed anti-TNF therapy, and from them randomly selected from among the Internal Medicine medical doctors (the specialty which most commonly prescribed biological therapy in these hospitals) those who had at least, one weekly consultation of autoimmune disease and were familiar with the hospital prescription procedures. One hospital which had only one doctor prescribing was excluded.
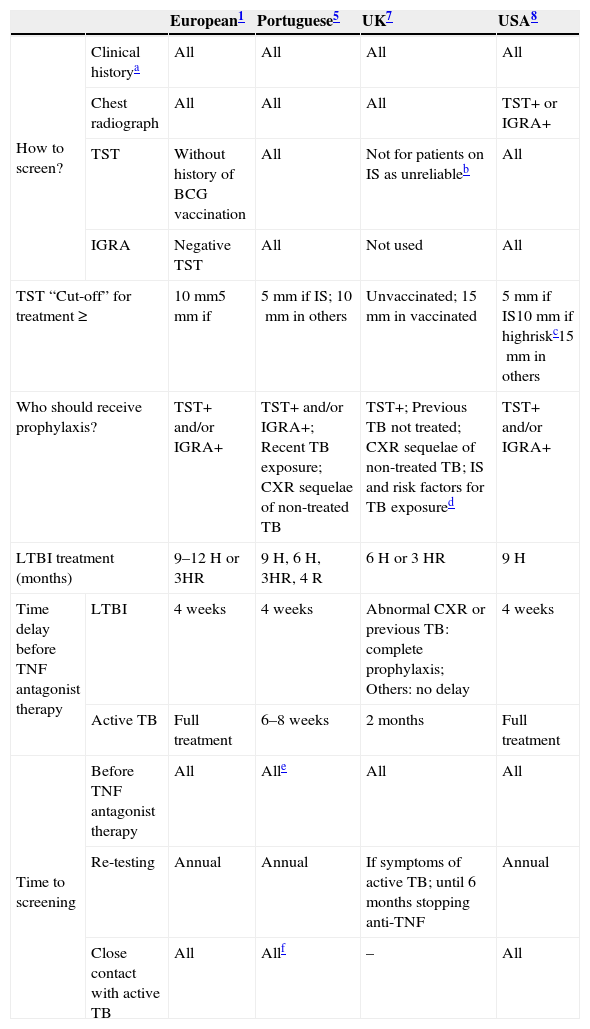
The enquiry was carried out in September 2012. The interview consisted of ten multiple-choice questions relating to each recommendation: TB screening, diagnostic exams, preventive treatment, when to start anti-TNFα therapy and TB monitoring through the course of anti-TNFα treatment. Compliance rates were determined for each clinical recommendation and each guideline [national, international or European consensus] (Table 1).5,1,6,7
Comparison between TB screening guidelines and the 2010 European consensus.
| European1 | Portuguese5 | UK7 | USA8 | ||
|---|---|---|---|---|---|
| How to screen? | Clinical historya | All | All | All | All |
| Chest radiograph | All | All | All | TST+ or IGRA+ | |
| TST | Without history of BCG vaccination | All | Not for patients on IS as unreliableb | All | |
| IGRA | Negative TST | All | Not used | All | |
| TST “Cut-off” for treatment ≥ | 10 mm5 mm if | 5 mm if IS; 10mm in others | Unvaccinated; 15mm in vaccinated | 5mm if IS10mm if highriskc15mm in others | |
| Who should receive prophylaxis? | TST+ and/or IGRA+ | TST+ and/or IGRA+; Recent TB exposure; CXR sequelae of non-treated TB | TST+; Previous TB not treated; CXR sequelae of non-treated TB; IS and risk factors for TB exposured | TST+ and/or IGRA+ | |
| LTBI treatment (months) | 9–12 H or 3HR | 9 H, 6 H, 3HR, 4 R | 6 H or 3 HR | 9H | |
| Time delay before TNF antagonist therapy | LTBI | 4 weeks | 4 weeks | Abnormal CXR or previous TB: complete prophylaxis; Others: no delay | 4 weeks |
| Active TB | Full treatment | 6–8 weeks | 2 months | Full treatment | |
| Time to screening | Before TNF antagonist therapy | All | Alle | All | All |
| Re-testing | Annual | Annual | If symptoms of active TB; until 6 months stopping anti-TNF | Annual | |
| Close contact with active TB | All | Allf | – | All | |
IS: immunosuppressive medication; H: isoniazid; R: rifampicin; CXR: chest radiograph.
History of prior TB infection and treatment, close contacts of persons known or suspected to have active TB.
TST will only become reliable once treatment has been stopped for 1 month in the case of steroids and for 3 months in the case of all other immunosuppressive drugs.
Risk factors for TB exposure are defined based on a publication from the US Centres for Disease Control and Prevention as: close contacts of persons known or suspected to have active TB; foreign-born persons from areas that have a high incidence of active TB (e.g., Africa, Asia, Eastern Europe, Latin America, and Russia); persons who visit areas with a high prevalence of active TB, especially if the visits are frequent or prolonged; residents and employees of congregate settings whose clients are at an increased risk for active TB (e.g., correctional facilities, long-term care facilities, and homeless shelters); health care workers who serve clients who are at an increased risk for active TB; populations defined locally as having an increased incidence of latent M. tuberculosis infection or active TB, possibly including medically underserved, low-income populations, or persons who abuse drugs or alcohol; and infants, children, and adolescents exposed to adults who are at an increased risk for latent M. tuberculosis infection or active.
Eleven hospitals were identified and ten were eligible. They performed TB screening in all patients treated with anti-TNF therapy. The screening method used by all hospitals was patient history and tuberculin skin test (TST), seven (70%) used interferon-gamma-release-assay (IGRA) and six (60%) used chest radiography (CRX). Compliance rate: 60% for UK guidelines and 50% for European consensus, USA and national guidelines (Table 2).
Summary of results and compliance to recommendations (results in percentages).
| European | National | UK | American | |
|---|---|---|---|---|
| Screen tools | 50 | 50 | 60 | 50 |
| Screen time | 75 | 75 | 90 | 75 |
| TST “cut-off” | 90 | 80 | 80 | 80 |
| Diagnosis methods of LTBI | 70 | 70 | 0 | 70 |
| LTBI treatment | 80 | 80 | 20 | 80 |
| Timing of initiation of anti-TNF if LTBI | 80 | 80 | 0 | 80 |
| TB re-testing | 10 | 10 | 0 | 10 |
All hospitals performed TB screening prior to anti-TNF treatment (compliance rate of 100% with all guidelines); seven (70%) re-screened during anti-TNF treatment, due to recent exposure to TB (compliance rate of 70% for USA, national guidelines and European consensus, UK guidelines do not give specific recommendations on this subject) and eight hospitals (80%) re-screened due to symptoms (compliance rate of 80% with all guidelines) (Table 2). Only one hospital (10%) re-tested asymptomatic patients with a previous negative screening, without recent exposure, during anti-TNF treatment (compliance rate 10% with national, American and European consensus, not applicable to UK guidelines) (Table 2).
Three hospitals (30%) screened in their own facilities, 4 (40%) in TB outpatient clinic, 1 (10%) in a primary care center and 2 (20%) in elsewhere.
Eight hospitals (80%) used TST cut-off of 5mm, one (10%) 10mm and one (10%) 15mm. The compliance rate was 80% percent with national, UK and USA guidelines and 90% with European consensus (Table 2).
Seven hospitals (70%) initiated preventive therapy, if TST or IGRA were positive; one (10%) if both TST and IGRA were positive; two (20%) if TST was positive, without IGRA result. A compliance rate of 70% with European consensus, national and north-American guidelines was calculated. No compliance for UK guidelines (Table 2). All centers used isoniazid for preventive treatment. Eight hospitals (80%) treated their patients during nine months and two (20%) during 6 months. Compliance rates: 80% with national, American and European consensus and 20% with UK guidelines (Table 2). Eight hospitals (80%) initiated anti-TNF therapy 4 weeks after beginning LTBI treatment and two (20%) 3 months after. Compliance rate: 80% with national, American and European consensus; no compliance with UK guidelines (Table 2).
TB screening is performed routinely before anti-TNF treatment, according to the individual physician's awareness of TB risk. In our study, the European consensus was the guideline with highest percentage of compliance, presenting a medium rate of 69.4%%, followed closely by National and American Guidelines (68.1%). UK recommendations had a lower adherence, with 64.3%. This rate is similar to the compliance rate observed by Smith et al. in the Gastroenterology and Dermatology G5 group (including the five foremost industrialized economies – Germany, France, Italy, Spain and the United Kingdom); it was lower than the one observed in the non-G5 Rheumatology group.8
Although this study has serious limitations, as TB screening performance was based on self-reported actions of the physicians concerned, we found that among the protocols followed by experienced anti-TNF prescribers, the rate of compliance with the guidelines is low and there is excessive confidence in the TST. Re-testing is neglected and is a something that needs to be improved. The availability of facilities in Portugal such as the TB outpatient clinic can be considered a real asset but they are not used to their full potential.
Conflicts of interestThe authors have no conflicts of interest to declare.