Coexistence between pulmonary cancer and chronic obstructive pulmonary disease (COPD) is frequent and is anticipated to be lead to high worldwide mortality in the next decades. The most powerful therapeutic approach for non-small cell lung carcinoma is lung surgical resection. However, in COPD patients, this approach bears a higher mortality and morbidity risk, thus requiring an accurate pre-operatory evaluation of the surgical risk comprising a clinical and functional assessment at rest, as well as a cardiopulmonary exercise test. In this observational study, factors associated with cardiopulmonary complications within 30 days after tumor resection surgery were investigated in a cohort of patients with COPD and lung cancer assigned to perform a cardiopulmonary exercise test.
This study included 50 patients (46 men, 92.0%) with a mean age of 64.7 years old (standard deviation 7.9), forced expiratory volume in the first second (FEV1) of 61.8% (SD 19.0%) and carbon monoxide diffusing capacity (DLCO) of 46.0% (SD 14.8%).
Complications were observed in eighteen patients (36.0%) including 2 deaths (4.0%). Peak oxygen uptake (VO2peak) expressed in percentage of the predicted value was the only parameter showing a statistically significant difference between the groups with and without complications (p=0.027). The best value of VO2peak to discriminate complications occurrence was 61.0%.
This study highlights the relevance of the cardiopulmonary exercise test in the risk assessment of pulmonary resection surgery in patients with COPD. The VO2peak (percentage of predicted value) is shown to be associated with complications within 30 days after surgery.
Chronic obstructive pulmonary disease (COPD) and lung cancer are two important causes of mortality and morbidity around the world. Lung cancer is a leading cause of cancer mortality in both genders.1 It is estimated that COPD will be the fourth worldwide leading cause of death in 2030.2 Since these two diseases occur frequently associated, it is expected that they will be major causes of death in developed countries.3
The most powerful therapeutic approach for non-small cell lung carcinoma is lung surgical resection.4 However, this option is associated with higher morbidity and mortality in patients with low ventilatory reserve, which is a common limiting factor for lung cancer surgery in patients with COPD.5,6 In this way, preoperative risk assessment should be performed to achieve an accurate risk stratification. Many parameters have been proposed as predictors of perioperative risk. They include clinical parameters: smoking, cough, wheeze, bronchorrhea and comorbidities, mainly cardiovascular7; functional parameters at rest: forced expiratory volume in the first second (FEV1) and diffusing capacity of the lung for carbon monoxide (DLCO); predicted postoperative FEV1 (ppoFEV1) and DLCO (ppoDLCO) values as well as, exercise tests: stair climbing test, shuttle walk test and/or cardiopulmonary exercise test (CPET) on cycle ergometer or treadmill.8 Exercise testing has been considered a better predictor of postoperative complications than rest evaluations and the CPET is the gold standard. This test assesses the physiological reserve at maximal exertion and, in this way, mimics the response to surgical stress or adverse post-operative events.8
In CPET, peak oxygen uptake (VO2peak) is the single most important parameter in the assessment of the surgery risk, with values above 20mL/kg/min or 75% of predicted being indicated as the limits for an acceptable risk for pneumonectomy.9 Although VO2peak values between 10–20mL/kg/min and/or 35–75% of predicted also allow lung resection surgery, it is necessary to evaluate with higher accuracy the risk of surgery within those intervals. As an example, Smith and Data proposed the cut-off VO2peak 15mL/kg/min.10,11
Our study was carried out to assess factors associated with cardiopulmonary complications within 30 days after tumor resection surgery, in a cohort of patients with COPD and lung cancer assigned to perform a CPET.
MethodsSubjectsIn the period between 1997 and 2012, a historical cohort of 50 consecutive patients with COPD and potentially resectable lung cancer were referred to perform CPET for risk stratification evaluation and afterwards were subject to tumor resection surgery at Serviço de Cirurgia Torácica, Hospital Pulido Valente, Lisbon, Portugal. The sample included all COPD patients considered to be at high surgical risk by their assistant surgeon. Criteria were clinical cardiopulmonary risk factors, such as being a current smoker, presenting bronchorrhea, dyspnea, exercise intolerance and relevant comorbidities, including cardiac, cerebrovascular and metabolic diseases; and/or respiratory functional limitation: FEV1 and/or DLCO below 60% predicted.11–13 Patients were characterized by age, gender, smoking history, comorbidities, lung cancer histology, neoadjuvant chemotherapy, extent of lung resection and cardiopulmonary complications within 30 days after surgery. All subjects’ clinical data were obtained from the medical records.
The hospital's ethics committee and administration board approved the conduction of the trial (IRB: DIRCLIN-22.NOV.2014-0487).
Lung function studySpirometry and diffusion capacity study were performed according to guidelines,14,15 as well as arterial blood gas samples. Predicted postoperative values for FEV1 and DLCO were calculated according to the segment method (based on the proportion of open and functional lung segments to be removed).9,16
Cardiopulmonary exercise testPatients underwent a symptom-limited cycle ergometry, following Wasserman et al.17 and American Thoracic Society/American College of Chest Physicians statement.18 The VO2peak value was recorded in L/min, mL/kg/min and as a percentage of predicted normal values according to Jones and Hansen equations.19,20
Pulmonary rehabilitationAll patients followed a postoperative pulmonary rehabilitation program that included lung expansion techniques, pain control and exercise training. Whenever possible, a preoperative intervention was also implemented, including smoking cessation interventions, respiratory physiotherapy, and exercise training.21
Statistical analysisAn exploratory analysis was performed for all variables. Categorical data were presented as frequencies and percentages, and continuous variables were presented as mean or median, standard deviation (SD), or interquartile range (IQR: 25th percentile–75th percentile) as appropriate.
The entire group was divided into patients with and without cardiopulmonary complications within 30 days after surgery. To compare sociodemographic, clinical, as well as rest and exercise functional data between the two groups, Student's t test and non-parametric tests (Chi-square test, Fisher's exact test, Mann–Whitney U test) were employed.
To categorize VO2peak levels, the minimum p-value approach was used, which provides the point from a grid of marker values that is associated with the minimum Chi-squared p-value.22 To evaluate the association between VO2peak and ppoFEV1, the nonparametric smoother LOWESS (Locally Weighted Scatterplot Smoother) was used.
The 95% confidence intervals (CI) were calculated whenever appropriate. A significance level α=5% was considered. All data were analyzed using the Statistical Package for the Social Sciences for Windows 21.0 (IMB Corp. Released 2012. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corporation) and the R software [R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, year=2014, http://www.R-project.org].
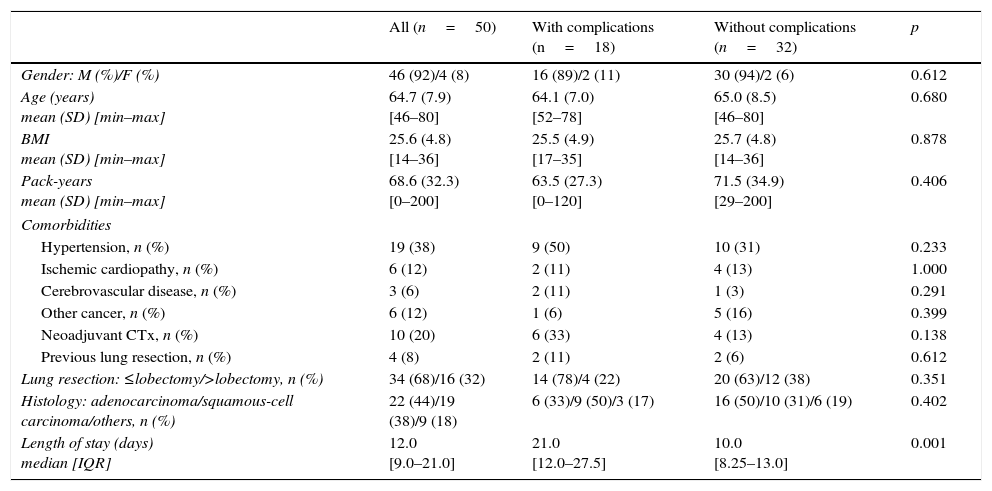
ResultsDuring the period studied, a total of 1674 lung resection surgeries for lung cancer were performed. This included 50 COPD patients who performed CPET based on clinical or functional criteria. Forty-six patients (92.0%) were male with a mean age of 64.7 years (SD 7.9) (range: 46–80 years). The most common comorbidities were arterial hypertension (19 patients, 38.0%), ischemic heart disease (6 patients, 12.0%), other neoplasm (6 patients, 12.0%) and cerebrovascular disease (3 patients, 6.0%) (Table 1). Twenty-five patients (50.0%) were still smoking one month before surgery.
Clinical and demographic data in the patients with and without cardiopulmonary complications within 30 days after tumor resection surgery (n=50).
| All (n=50) | With complications (n=18) | Without complications (n=32) | p | |
|---|---|---|---|---|
| Gender: M (%)/F (%) | 46 (92)/4 (8) | 16 (89)/2 (11) | 30 (94)/2 (6) | 0.612 |
| Age (years) mean (SD) [min–max] | 64.7 (7.9) [46–80] | 64.1 (7.0) [52–78] | 65.0 (8.5) [46–80] | 0.680 |
| BMI mean (SD) [min–max] | 25.6 (4.8) [14–36] | 25.5 (4.9) [17–35] | 25.7 (4.8) [14–36] | 0.878 |
| Pack-years mean (SD) [min–max] | 68.6 (32.3) [0–200] | 63.5 (27.3) [0–120] | 71.5 (34.9) [29–200] | 0.406 |
| Comorbidities | ||||
| Hypertension, n (%) | 19 (38) | 9 (50) | 10 (31) | 0.233 |
| Ischemic cardiopathy, n (%) | 6 (12) | 2 (11) | 4 (13) | 1.000 |
| Cerebrovascular disease, n (%) | 3 (6) | 2 (11) | 1 (3) | 0.291 |
| Other cancer, n (%) | 6 (12) | 1 (6) | 5 (16) | 0.399 |
| Neoadjuvant CTx, n (%) | 10 (20) | 6 (33) | 4 (13) | 0.138 |
| Previous lung resection, n (%) | 4 (8) | 2 (11) | 2 (6) | 0.612 |
| Lung resection: ≤lobectomy/>lobectomy, n (%) | 34 (68)/16 (32) | 14 (78)/4 (22) | 20 (63)/12 (38) | 0.351 |
| Histology: adenocarcinoma/squamous-cell carcinoma/others, n (%) | 22 (44)/19 (38)/9 (18) | 6 (33)/9 (50)/3 (17) | 16 (50)/10 (31)/6 (19) | 0.402 |
| Length of stay (days) median [IQR] | 12.0 [9.0–21.0] | 21.0 [12.0–27.5] | 10.0 [8.25–13.0] | 0.001 |
M: male; F: female; BMI: body mass index; CTx: chemotherapy; IQR: 25th percentile–75th percentile; max: maximum; min: minimum; SD: standard deviation.
The mean FEV1 was 1.73L/min (SD: 0.50; ranging from 0.91 to 3.04L/min) which corresponded to 61.8% of predicted (SD: 9.0%; 33–106%). DLCO was evaluated in 21 patients with a mean of 46.0% (SD: 14.8%; 27–71%). Of all patients, 36 (72.0%) had FEV1 and/or DLCO below 60%. The mean ppoFEV1 was 42.7% (SD: 14.1; 22–88%) and mean ppoDLCO was 30.4% (SD: 11.3; 11–52%).
Thirty-five patients (70.0%) underwent lobectomy or bilobectomy, 11 pneumonectomy (22.0%) and 4 (8.0%) wedge lung resection.
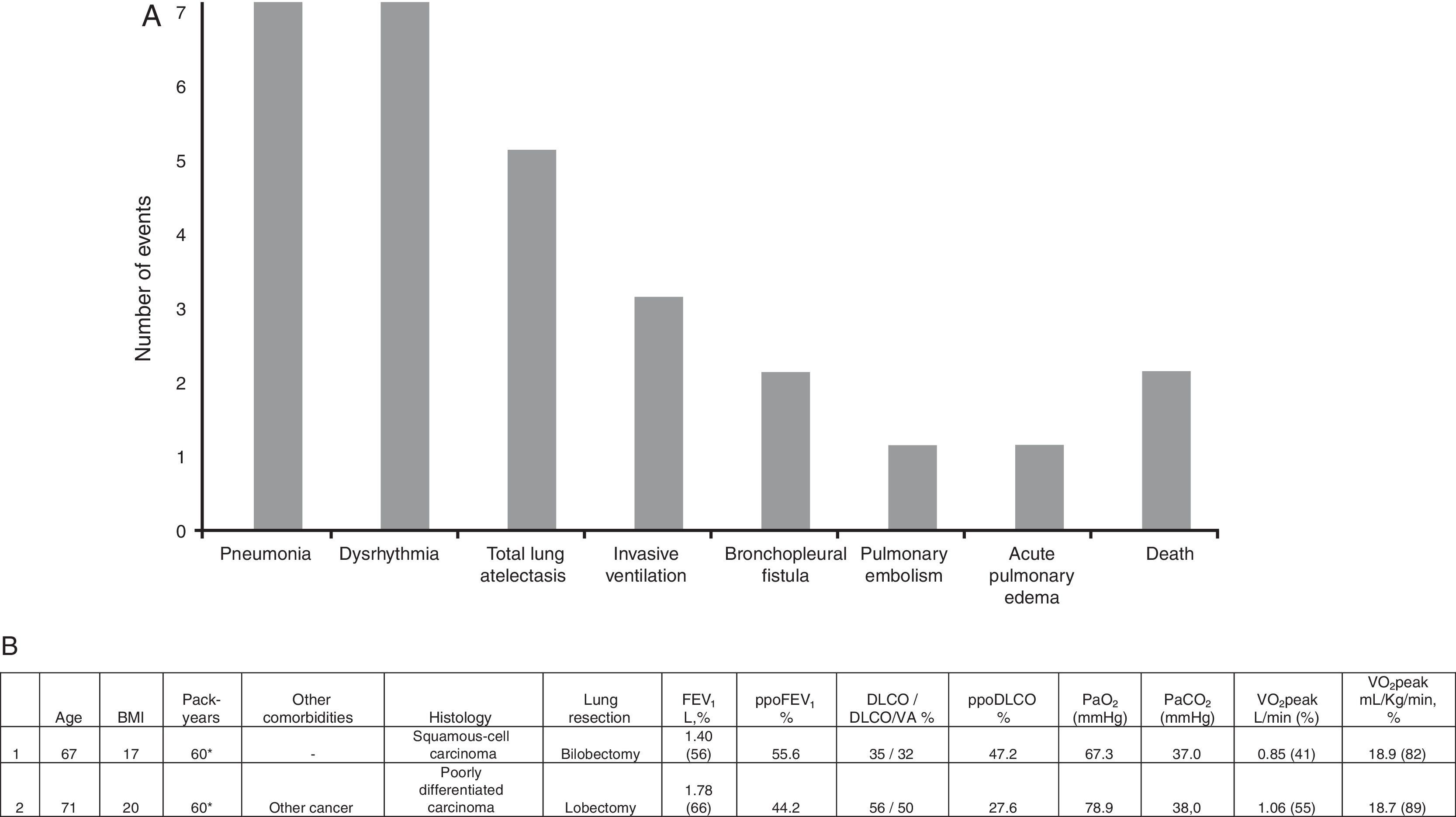
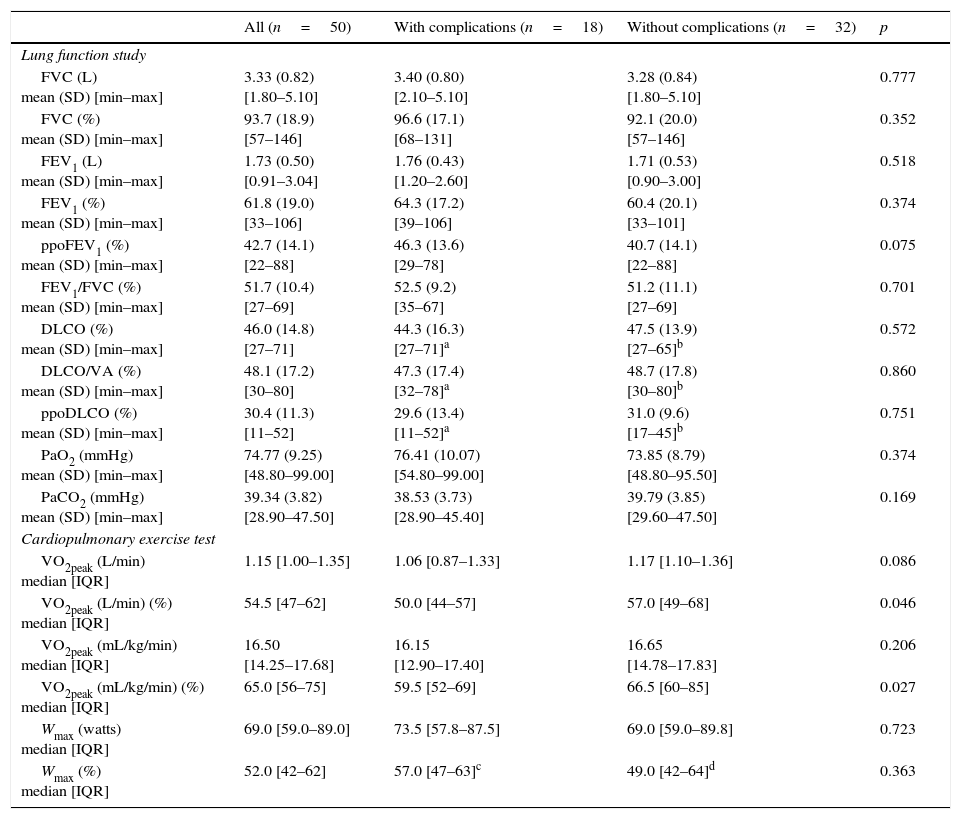
Eighteen patients (36.0%) had one or more cardiopulmonary complications within 30 days after tumor resection surgery, including 2 deaths (4.0%). The most frequent complications were pneumonia (n=7; 38.9%), dysrhythmia (n=7; 38.9%) and total lung atelectasis (n=5; 27.8%), as shown in Fig. 1A. Death occurred in two patients submitted to lobectomy and bilobectomy, attributed to postoperative pneumonia requiring invasive ventilation. Fig. 1B summarizes sociodemographic, clinical and functional characteristics of the deceased patients. The rate of pneumonia mortality was 28.7%.
(A) Cardiorespiratory complications within 30 days after tumor resection surgery. (B) Clinical, demographic and functional data of patients who died. *Current smoker. BMI: body mass index; FEV1: forced expiratory volume in the first second; ppoFEV1: predicted postoperative value for FEV1; DLCO: carbon monoxide diffusing capacity; DLCO/VA: DLCO corrected to alveolar volume; ppoDLCO: predicted postoperative value for DLCO; PaO2: partial pressure of arterial oxygen; PaCO2: partial pressure of arterial carbon dioxide; VO2peak: peak oxygen uptake.
The mean length of stay was 15 days (SD: 8; 5–36 days), being statistically significant longer in the group with complications (20.3; SD: 8.4 versus 12.1; SD: 6.4 days, p<0.001).
There was no statistically significant difference in sociodemographic and clinical variables evaluated within the groups with and without complications (Table 1).
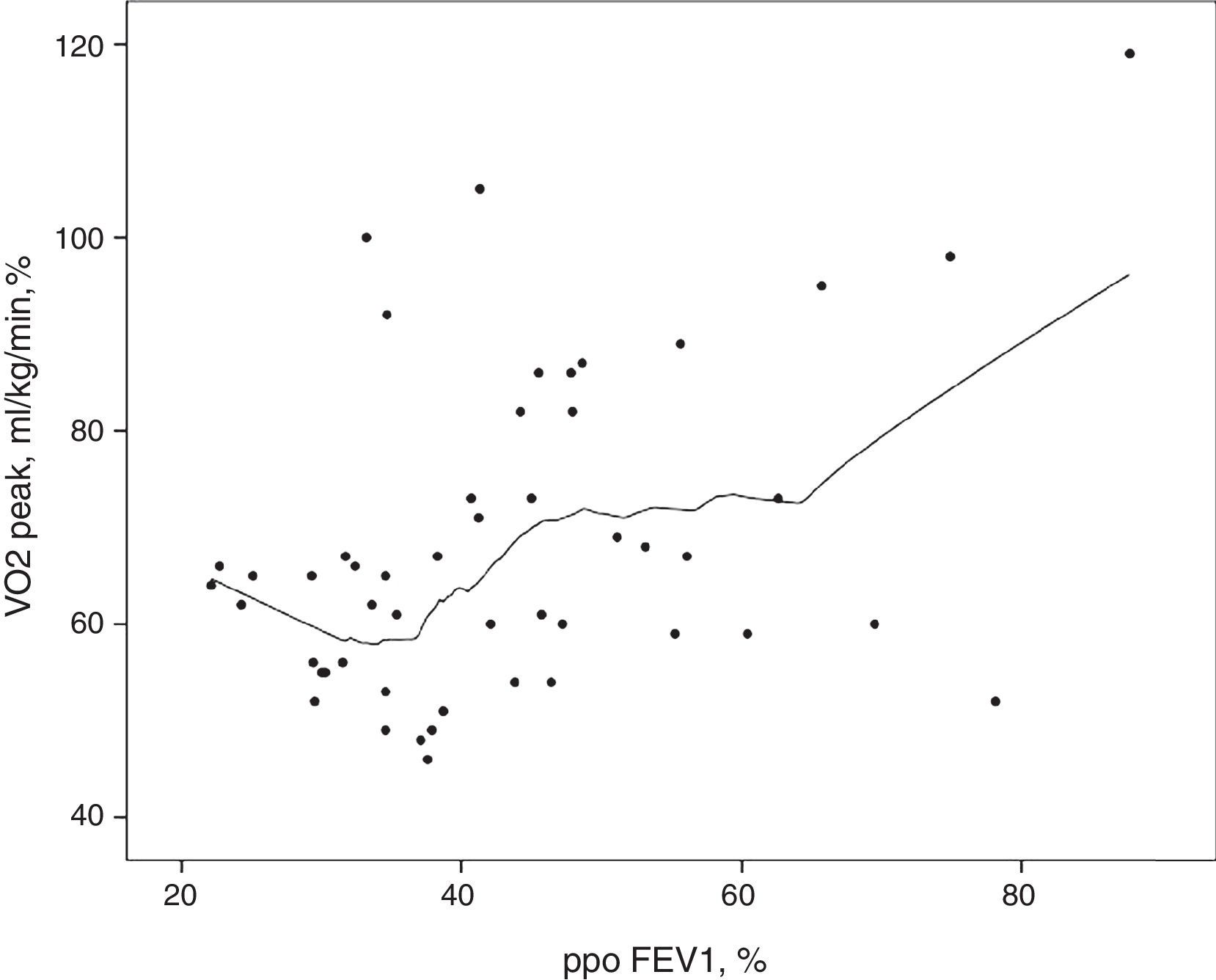
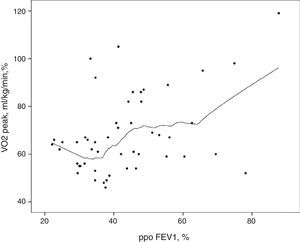
Likewise, pulmonary function at rest did not evidence significantly differences between the groups (Table 2). There was no direct association between ppoFEV1 and VO2peak. As shown in Fig. 2, patients with lower ppoFEV1 values (between 22.1% and 38.7%) had a wide range of VO2peak values (46–100%).
Lung function study at rest and cardiopulmonary exercise test in patients with and without cardiopulmonary complications within 30 days after tumor resection surgery (n=50).
| All (n=50) | With complications (n=18) | Without complications (n=32) | p | |
|---|---|---|---|---|
| Lung function study | ||||
| FVC (L) mean (SD) [min–max] | 3.33 (0.82) [1.80–5.10] | 3.40 (0.80) [2.10–5.10] | 3.28 (0.84) [1.80–5.10] | 0.777 |
| FVC (%) mean (SD) [min–max] | 93.7 (18.9) [57–146] | 96.6 (17.1) [68–131] | 92.1 (20.0) [57–146] | 0.352 |
| FEV1 (L) mean (SD) [min–max] | 1.73 (0.50) [0.91–3.04] | 1.76 (0.43) [1.20–2.60] | 1.71 (0.53) [0.90–3.00] | 0.518 |
| FEV1 (%) mean (SD) [min–max] | 61.8 (19.0) [33–106] | 64.3 (17.2) [39–106] | 60.4 (20.1) [33–101] | 0.374 |
| ppoFEV1 (%) mean (SD) [min–max] | 42.7 (14.1) [22–88] | 46.3 (13.6) [29–78] | 40.7 (14.1) [22–88] | 0.075 |
| FEV1/FVC (%) mean (SD) [min–max] | 51.7 (10.4) [27–69] | 52.5 (9.2) [35–67] | 51.2 (11.1) [27–69] | 0.701 |
| DLCO (%) mean (SD) [min–max] | 46.0 (14.8) [27–71] | 44.3 (16.3) [27–71]a | 47.5 (13.9) [27–65]b | 0.572 |
| DLCO/VA (%) mean (SD) [min–max] | 48.1 (17.2) [30–80] | 47.3 (17.4) [32–78]a | 48.7 (17.8) [30–80]b | 0.860 |
| ppoDLCO (%) mean (SD) [min–max] | 30.4 (11.3) [11–52] | 29.6 (13.4) [11–52]a | 31.0 (9.6) [17–45]b | 0.751 |
| PaO2 (mmHg) mean (SD) [min–max] | 74.77 (9.25) [48.80–99.00] | 76.41 (10.07) [54.80–99.00] | 73.85 (8.79) [48.80–95.50] | 0.374 |
| PaCO2 (mmHg) mean (SD) [min–max] | 39.34 (3.82) [28.90–47.50] | 38.53 (3.73) [28.90–45.40] | 39.79 (3.85) [29.60–47.50] | 0.169 |
| Cardiopulmonary exercise test | ||||
| VO2peak (L/min) median [IQR] | 1.15 [1.00–1.35] | 1.06 [0.87–1.33] | 1.17 [1.10–1.36] | 0.086 |
| VO2peak (L/min) (%) median [IQR] | 54.5 [47–62] | 50.0 [44–57] | 57.0 [49–68] | 0.046 |
| VO2peak (mL/kg/min) median [IQR] | 16.50 [14.25–17.68] | 16.15 [12.90–17.40] | 16.65 [14.78–17.83] | 0.206 |
| VO2peak (mL/kg/min) (%) median [IQR] | 65.0 [56–75] | 59.5 [52–69] | 66.5 [60–85] | 0.027 |
| Wmax (watts) median [IQR] | 69.0 [59.0–89.0] | 73.5 [57.8–87.5] | 69.0 [59.0–89.8] | 0.723 |
| Wmax (%) median [IQR] | 52.0 [42–62] | 57.0 [47–63]c | 49.0 [42–64]d | 0.363 |
FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; ppoFEV1: predicted postoperative value for FEV1; DLCO: carbon monoxide diffusing capacity; DLCO/VA: DLCO corrected to alveolar volume; ppoDLCO: predicted postoperative value for DLCO; PaO2: partial pressure of arterial oxygen; PaCO2: partial pressure of arterial carbon dioxide; VO2peak: peak oxygen uptake; W: work rate. IQR: 25th percentile–75th percentile; max: maximum; min: minimum; SD: standard deviation.
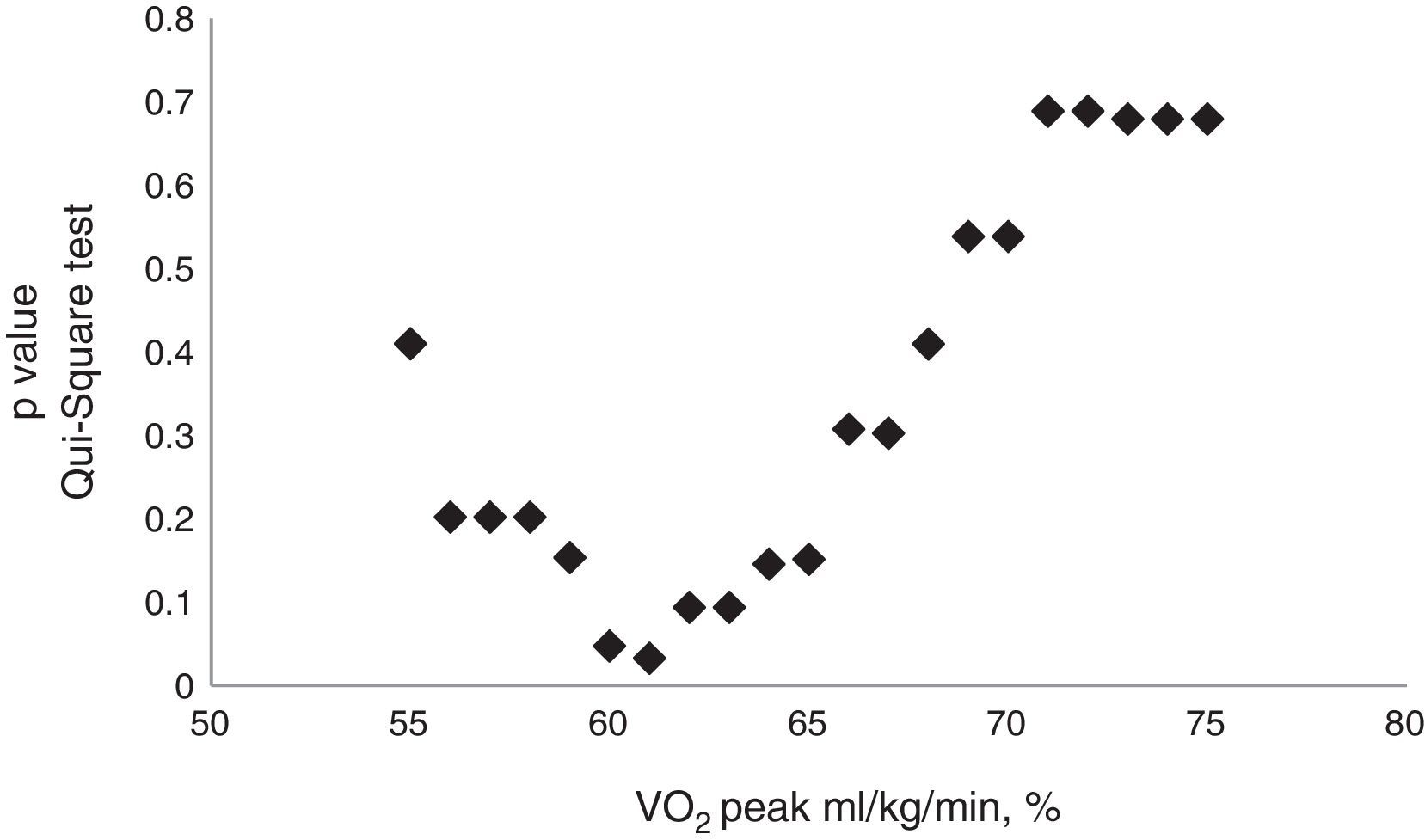
There was a statistically significant difference in VO2peak value when it was expressed in percentage of predicted mL/kg/min value (59.5 versus 66.5%) and L/min (50.0 versus 57.0%), between patients with and without complications (Table 2). The achieved cut-off point which defined the group with higher number of complications was 61.0% (Fig. 3). Among patients with complications, 12 (66.7%) had VO2peak (mL/kg/min) below or equal to 61.0% and only 6 (33.3%) had values above this cut-off [OR: 5.1 (1.5; 17.8), p=0.010].
In relation to the VO2peak absolute values, fifteen patients (30.0%) had VO2peak lower than 15mL/kg/min, from whom, 7 (46.7%) had one or more complications, however, without mortality. In this sample, VO2peak cut-off of 15mL/kg/min did not distinguish the patients with or without complications (p=0.304). In contrast to VO2peak expressed in percentage of normal values, the difference documented in VO2peak absolute value did not show statistical significance (Table 2).
DiscussionThis study highlights the relevance of CPET in the risk stratification of lung cancer resection surgery in patients with COPD. Among these high risk patients, VO2peak was the only parameter associated to postoperative complications, which is consistent with other studies.9,10,12,23
The present study underlines the VO2peak expressed in percentage of predicted normal values and indicates the cut-off of 61.0% below which, there was a significant increase of postoperative complications. Other authors also highlight VO2peak percentage of predicted values as a better predictor of postoperative complications than the absolute values. The latter does not take gender, age and height into account.12,23 Bolliger et al. proposed the cut-off value below 60% of predicted as being highly predictive of complications.12 They also considered that values of VO2peak above 75% of predicted values indicate low risk of complications, regardless of the extent of lung resection. Win et al. suggested a VO2peak threshold of 50–60% of predicted as an acceptable indication for lung resection.23 Cut-off values of VO2peak expressed in mL/kg/min have also been suggested. Thus, VO2peak above 20mL/kg/min have been indicated as the limits for acceptable risk for pneumonectomy9 and values below 10mL/kg/min as very high risk for any kind of lung resection.8
However, the available scientific evidence is still insufficient to define the VO2peak threshold for lobectomy.8 Considering that in our study most of the resections (78.0%) were less radical than pneumonectomy (70.0% lobectomy or bilobectomy and 8.0% wedge resections), we believe that our data contributed to establish the VO2peak threshold for the lobectomy complications.
Apart from discriminative value of the CPET for complications, it also meant that in our sample high-risk patients (according to clinical and/or resting lung function parameters) underwent curative-intent lung cancer surgery with higher life expectancy and an acceptable mortality. In fact, among the 36 patients with FEV1 and/or DLCO less than 60%, the CPET allowed the tumor resection in 34 patients (94.4%).
The results of this study indicate that CPET is a more reliable global assessment tool than lung function testing performed at rest. The superior value of VO2peak compared to ppoFEV1, is shown in Fig. 2, where the lowest ppoFEV1 values were associated with a wide range of VO2peak values.
The rate of complications (36.0%) and mortality (4.0%) were similar to other authors’ results – 20–40% of complications and 1.6–7% of mortality.24
In our study, the cause of the two deaths was pneumonia requiring invasive ventilation. Pneumonia is one of the most common complications after lung resection surgery and, in Portugal, it is associated with high intra hospital mortality (20.4% from 2000 to 2009),25 particularly in patients above 65 years.26
Vaporciyan et al.27 showed that in patients submitted to pneumonectomy, the incidence of pulmonary complications, mainly pneumonia and acute respiratory distress syndrome, was greater in those who continued to smoke up to one month before surgery. Arguing that the occurrence of pulmonary complications was associated with higher mortality, these authors suggest the implementation of interventions to prevent them. American College of Chest Physicians21 and European Respiratory Society/European Society of Thoracic Surgery8 recommend smoking cessation at the time of lung cancer diagnosis and for at least 2–4 weeks before surgery, as a way of improving lung surgery outcomes.
The present study underlines the importance of accurate lung cancer resection risk stratification in patients with COPD, allowing a curative-intent lung cancer surgery without significant mortality and morbidity. This study has some limitations that need to be addressed. We emphasize the low number of patients and the unknown DLCO value in a large number of patients. Despite these limitations, the sample is homogenous according to the two associated diseases (COPD and lung cancer) and the same team of thoracic surgeons performing all surgeries. These aspects decreased bias and variability and assigns clinical and scientific interest to our results.
As the criteria to perform CPET is a low FEV1 and/or low DLCO, which was present in the majority of our sample data (72%), these might have biased the association of lung function at rest (FEV1 and DLCO) and the complications, not showing a significant correlation.
ConclusionCPET is a valuable tool in the risk assessment of pulmonary resection surgery in patients with lung cancer and COPD. Among these high-risk patients, regarding clinical and functional parameters, VO2peak expressed in percentage of the predicted value was the best discriminator for occurrence of complications and should be taken into account in the assessment of lung resection surgery risk.
This study contributed to define 61% value as the VO2peak cut-off above which lung resection surgery risk is considered acceptable in patients with lung cancer and COPD.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors’ contributionFatima Rodrigues and Monica Grafino shared the first authorship of the manuscript. Nevertheless, all authors gave a substantial contribution to the manuscript in the conception, study design, data collection and results interpretation. Ana Luísa Papoila also carried out the statistical analysis of the data, and reviewed all manuscript versions. All authors approved the manuscript and its submission.
This study was carried out at the Serviço de Pneumologia and Serviço de Cirurgia Torácica, CHLN-Hospital Pulido Valente. Alameda das Linhas de Torres, 117 1769-001 Lisbon, Portugal.



![Cut-off point value of VO2peak, mL/kg/min (61%) distinguished patients’ group with higher number of complications versus those with less complications (67% versus 33%) (OR: 5.1 [1.5; 17.8], p=0.010). Cut-off point value of VO2peak, mL/kg/min (61%) distinguished patients’ group with higher number of complications versus those with less complications (67% versus 33%) (OR: 5.1 [1.5; 17.8], p=0.010).](https://static.elsevier.es/multimedia/21735115/0000002200000005/v1_201609070038/S2173511516300161/v1_201609070038/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)



