Adherence to controller medication is a major problem in asthma management, being difficult to assess and tackle. mHealth apps can be used to assess adherence. We aimed to assess the adherence to inhaled corticosteroids+long-acting β2-agonists (ICS+LABA) in users of the MASK-air® app, comparing the adherence to ICS+formoterol (ICS+F) with that to ICS+other LABA.
Materials and methodsWe analysed complete weeks of MASK-air® data (2015-2022; 27 countries) from patients with self-reported asthma and ICS+LABA use. We compared patients reporting ICS+F versus ICS+other LABA on adherence levels, symptoms and symptom-medication scores. We built regression models to assess whether adherence to ICS+LABA was associated with asthma control or short-acting beta-agonist (SABA) use. Sensitivity analyses were performed considering the weeks with no more than one missing day.
ResultsIn 2598 ICS+LABA users, 621 (23.9%) reported 4824 complete weeks and 866 (33.3%) reported weeks with at most one missing day. Higher adherence (use of medication ≥80% of weekly days) was observed for ICS+other LABA (75.1%) when compared to ICS+F (59.3%), despite both groups displaying similar asthma control and work productivity. The ICS+other LABA group was associated with more days of SABA use than the ICS+F group (median=71.4% versus 57.1% days). Each additional weekly day of ICS+F use was associated with a 4.1% less risk in weekly SABA use (95%CI=-6.5;-1.6%;p=0.001). For ICS+other LABA, the percentage was 8.2 (95%CI=-11.6;-5.0%;p<0.001).
ConclusionsIn asthma patients adherent to the MASK-air app, adherence to ICS+LABA was high. ICS+F users reported lower adherence but also a lower SABA use and a similar level of control.
Suboptimal adherence is common in asthma.1 It is associated with poor control, increased risk of exacerbations and increased healthcare utilisation2, with an unnecessary increase of potentially harmful and/or expensive treatments.3 While assessing adherence in asthma is particularly relevant, it may be extremely challenging, given the need to capture individual day-to-day variability patterns.4,5
The assessment of adherence can be estimated using several methods.6 Such methods may involve the use of digital adherence technologies (DATs), which are digital systems used to aid adherence measurement and management. Electronic monitoring devices (EMDs, including smart inhalers) are DATs that directly and automatically measure the time and date of a dose being administered. They collect data on inhaler usage and transmit them through an app.6 EMDs are highly accurate7,8 but there is potential for dose dumping.9 Moreover, they are often associated with a single product and therefore (i) EMDs do not report the entire treatment10 and (ii) when patients switch their medication, they can no longer be used. Other DATs that have been used in asthma to assess adherence include mHealth apps without sensors.11–15 An observational cross-sectional study in rhinitis using the MASK-air® app has assessed the medication possession ratio (MPR) in 1887 users, of whom only 11% were adherent.16
Considering the relevance of proper adherence to asthma control medication, we used the MASK-air® app16 to investigate adherence to inhaled corticosteroids (ICS) + long-acting β2-agonists (LABA) as well as its association with asthma control. In particular, we compared patients treated with ICS+formoterol (ICS+F) versus ICS+other LABA, given the fact that, contrary to ICS+other LABA, ICS+F can not only be used as a maintenance therapy but also as a reliever treatment.
MethodsIn this longitudinal analysis, we analysed MASK-air® data from patients with self-reported asthma who reported complete weeks and at least one day of ICS use. We compared adherence levels in patients using ICS+F versus those using ICS+other LABA. In addition, we compared these groups on reported symptoms and symptom-medication scores, performing stratified analyses according to weekly adherence levels. Finally, we built regression models to assess whether the weekly use of ICS+F or ICS+other LABA was associated with the weekly use of short-acting beta-agonists (SABA).
Setting and participantsMASK-air® (www.mask-air.com) is freely available in 27 countries and can be downloaded via the Apple App and Google Play Stores.
We assessed MASK-air® users aged 16–75 years (or 13–75 years in countries with a lower age of digital consent), with self-reported asthma and who reported at least one day of ICS+F or ICS+other LABA use. Patients who reported both ICS+F and ICS+other LABA use were excluded. We analysed all weeks (sets of seven consecutive days) from May 2015 to December 2022 during which patients answered to the MASK-air® daily monitoring questionnaire on all days.17 For sensitivity analyses, we analysed (i) all weeks within the same period during which patients had at most one missing day of MASK-air® reporting and (ii) all months during which patients answered to the MASK-air® daily monitoring questionnaire on most days (i.e., having at most four missing days).
EthicsMASK-air® follows the General Data Protection Regulation.18 An independent review board approval was not required for this study as (i) the use of MASK-air® data for research purposes has been approved by an independent review board (Köln–Bonn, Germany),19 (ii) all data were anonymised before the study and (iii) users agreed to the analysis of their data in the terms of use (translated into all languages and customised according to the legislation of each country).
Data sources and variablesThe MASK-air® app comprises a daily monitoring questionnaire assessing (i) the daily asthma and rhinitis symptoms by means of 0–100 visual analogue scales (VASs) (e-Table 1) and (ii) asthma and rhinitis daily medication use available from country-specific lists.17 Information on the MASK-air® daily monitoring questionnaire allows for the computation of two symptom-medication scores: the combined symptom-medication score (CSMS)20 and the electronic daily control score for asthma (e-DASTHMA).21
Data analysisWhen responding to the MASK-air® daily monitoring questionnaire, it is not possible to skip any of the questions. Data are saved to the dataset only after the final answer, which precludes any missing data. All analyses were performed using the software R.
We computed effect size measures for all comparisons between weeks from patients under ICS+F versus ICS+other LABA (effect size helps to understand the magnitude of differences, whereas statistical significance examines whether the findings are likely to be due to chance. With large samples, p-values are very often significant, rendering it important to use the effect size). Values >0.2 were considered to represent meaningful differences (i.e., sufficiently large differences to be potentially relevant from a clinical point of view). Values of 0.2-0.5 were considered to represent small effect sizes, 0.5-0.8 medium effect sizes and >0.8 large effect sizes.22
We compared weeks reported by patients under ICS+F versus those reported by patients under ICS+other LABA on (i) weekly median and maximum VAS,17 CSMS20 and e-DASTHMA21 levels and (ii) adherence levels. We considered that there was medication adherence for the weeks when the self-reported use of ICS+F or ICS+other LABA occurred in >80% of the days (therefore, estimating adherence in an analogous way to the MPR).16 For weeks when adherence was not reached, we performed separate analyses for those in which the aforementioned medication (i) was not used, (ii) was used on 1-40% of the days and (iii) was used on 41-80% of the days.
We compared patients under ICS+F versus those under ICS+other LABA. Stratified analyses were performed, according to weekly adherence levels, on VAS asthma levels (both as a continuous variable and categorised according to its cut-offs), e-DASTHMA21 levels and frequency of SABA use.
We built mixed-effects linear regression models to compare, for both ICS+F and ICS+other LABA groups, VAS asthma levels on days when such medication was used versus those on which it was not used. Observations were clustered by patient (i.e., the patient was set as a random-effect). Analyses were performed considering (i) all weeks and (ii) weeks with adherence.
We built Poisson regression models23 to assess the association between weekly adherence to ICS+F or ICS+other LABA (independent variable) and number of days within a week with use of SABA (outcome variable). We built both univariable and multivariable regression models, with the latter involving an adjustment for weekly median VAS asthma levels.
ResultsIn MASK-air®, 9721 users had self-reported asthma. Of these users, 4753 reported at least one day of treatment: 1705 users (60,521 days) reported at least one day of ICS+F and 893 (26,396 days) at least one day of ICS+other LABA (e-Figure 1).
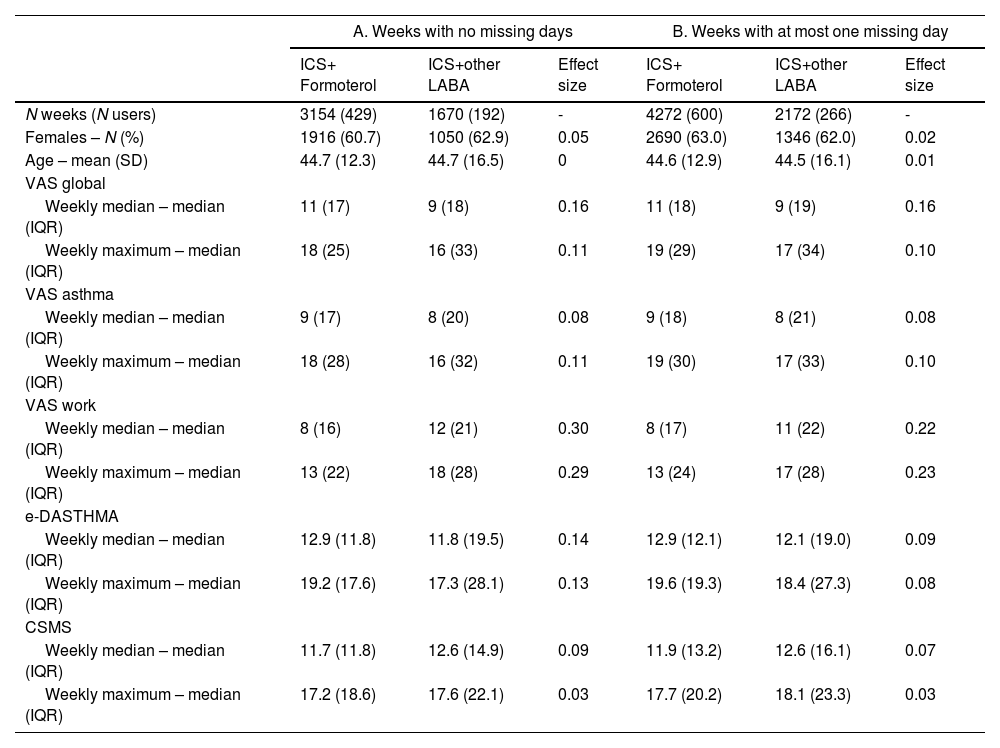
In our main analysis (no missing days per week), we analysed 4824 weeks (621 users, 23.9% of all ICS+LABA users), including 3154 weeks from 429 users under ICS+F and 1670 weeks from 192 users under ICS+other LABA (Table 1, e-Table 2, e-Fig. 1). In the sensitivity analysis assessing weeks with at most one missing day, we analysed 6444 weeks (866 users, 34.0% of all ICS+LABA users), including 4272 weeks from 600 users under ICS+F and 2172 weeks from 266 users under ICS+other LABA.
Characteristics of the main sample (A. weeks with no missing days) and of one of the samples assessed in sensitivity analysis (B. weeks with at most one missing day).
CSMS=Combined symptom-medication score; e-DASTHMA= Electronic daily control score for asthma; ICS=Inhaled corticosteroids; IQR=Interquartile range; LABA=Long-acting beta-agonists; VAS=Visual analogue scale.
Similar descriptive results were observed for full weeks and for weeks with at most one missing day (Table 1).
Users reporting ICS+F had a similar asthma control to those reporting ICS+other LABA for VAS global, VAS asthma and e-DASTHMA levels, with no meaningful differences observed (Table 1). Median VAS work was higher in the ICS+other LABA group (effect sizes=0.29–0.30).
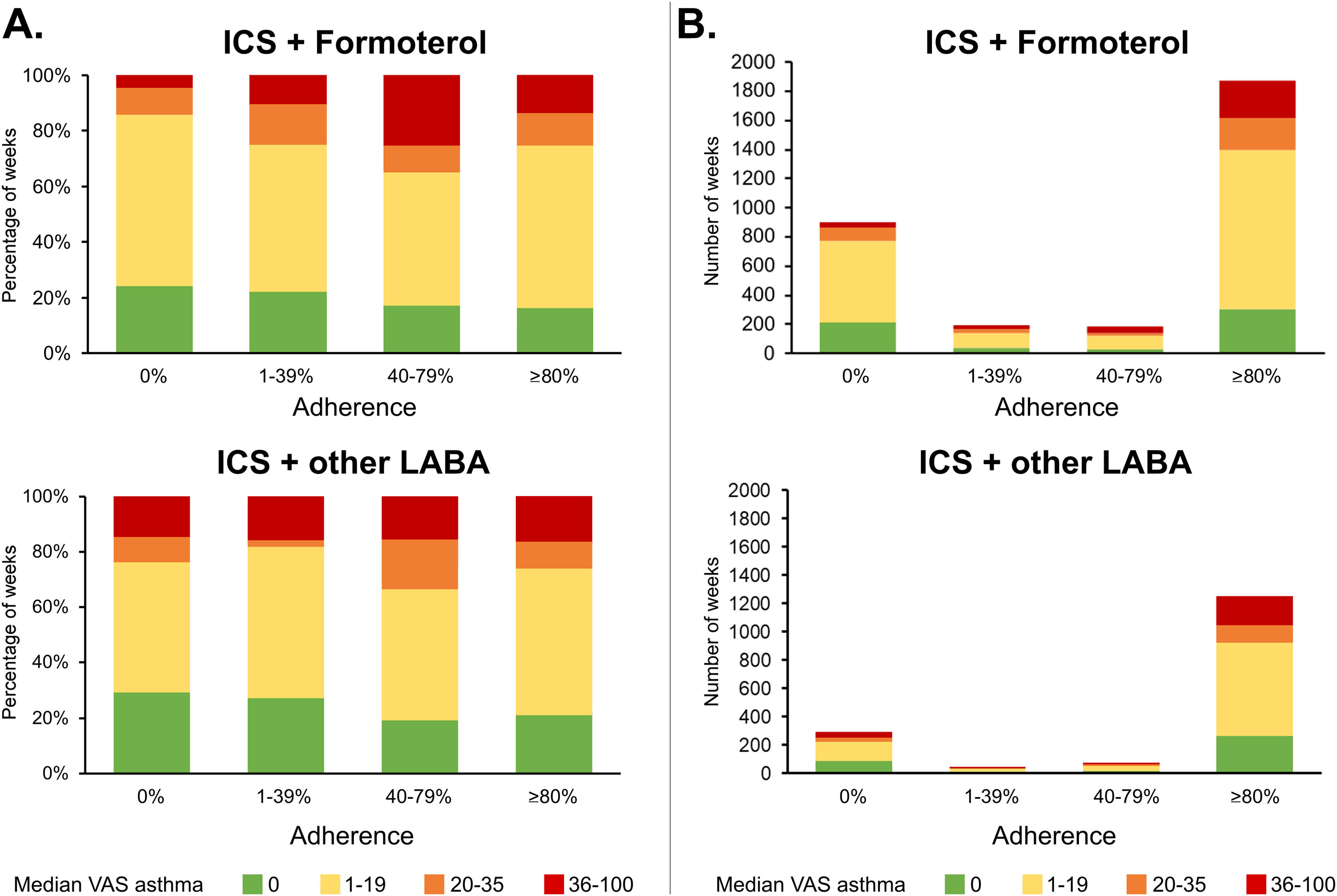
Adherence to asthma treatmentIn the main analysis, adherence (MPR>80%) was observed in 3125 weeks (64.8%). It was meaningfully higher in the ICS+other LABA group (75.1%) than in the ICS+F group (59.3%) (effect size=0.34) (e-Table 3). In 267 weeks (5.5%), there was partial adherence (MPR 41–80%), in 236 (4.9%) low adherence (MPR 1–40%) and in 1196 (24.8%) no adherence (MPR=0%) to ICS+LABA. We observed a bimodal adherence pattern, with most weeks associated with adherence or no adherence. VAS asthma and e-DASTHMA levels tended to be higher in ICS+F than in ICS+other LABA users (effect size range: 0.06–0.68, e-Table 3). Meaningful differences between both groups were not observed for weeks with adherence.
Considering VAS asthma cut-off values, uncontrolled asthma (VAS≥36/100) was mostly found in patients with at least partial adherence in both groups (e-Table 4; Fig. 1). Partly-controlled asthma (VAS 20-35/100) was found mostly in ICS+LABA-adherent patients and in adherent or totally non-adherent patients of the ICS+F group.
Similar results were observed in sensitivity analyses concerning weeks with at most one missing day of MASK-air® data. We also assessed 907 months with at most four missing days of MASK-air® reporting, with similar results observed (N users=214; e-Tables 5-7).
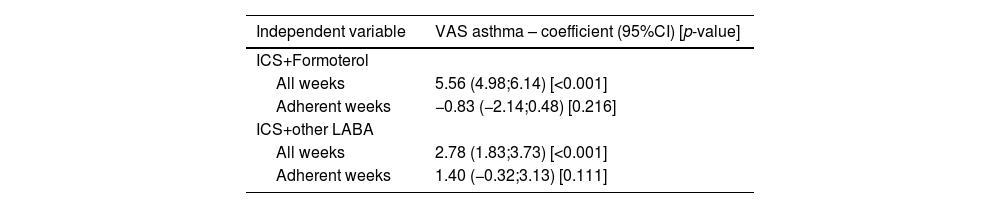
Days with and without ICS+LABAConsidering all weeks, higher VAS asthma levels were observed for days on which ICS+LABA were used versus those on which such medications were not used (Table 2). These associations were observed both for ICS+F (regression coefficient=5.6; 95%CI=5.0;6.1; p<0.001) and for ICS+other LABA (regression coefficient=2.8; 95%CI=1.8;3.7); p<0.001). However, these differences were not observed when only weeks with adherence (MPR>80%) were considered. Similar results were noted in sensitivity analyses (e-Table 8).
Results of linear regression models comparing days with versus without use of ICS + LABA on VAS asthma levels.
CI=Confidence interval; ICS=Inhaled corticosteroids; LABA=Long-acting beta-agonist; VAS=Visual analogue scale.
The number of weeks with SABA use was higher in ICS+other LABA users (23.3%) than in ICS+F users (15.5%; effect size=0.20, e-Table 3). Furthermore, in weeks with SABA use, there were more days of SABA use in ICS+other LABA (median=71.4% days) than in ICS+F users (median=57.1% days) (effect size=0.26). This trend was also found when analysing only weeks with adherence (MPR>80%) to ICS+LABA (median for ICS+F=57.1%, median for ICS+other LABA=71.4%, effect size=0.26) (e-Table 3).
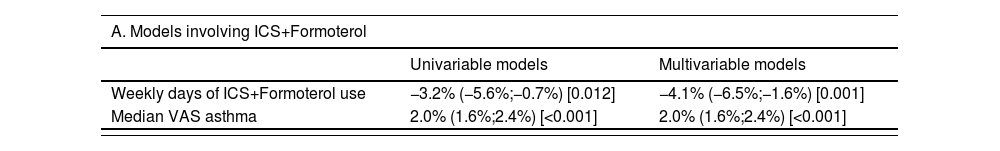
Increased adherence to ICS+F or ICS+other LABA was associated with lower SABA use, even after adjustment for VAS asthma levels. Each additional weekly day of ICS+F use was associated with a 4.1% average decrease in the risk of weekly SABA use (95%CI=−6.5%,−1.6%; p=0.001) compared to a 8.2% decrease with ICS+other LABA (95%CI=−11.6%,−5.0%; p<0.001) (Table 3).
Results of univariable and multivariable Poisson regression models modelling the percentage change in weekly SABA use per unit increase of the (i) number of weekly days of ICS+LABA use and/or (ii) median VAS asthma. Results are presented as percentage change (95% confidence intervals) [p-value].
ICS=Inhaled corticosteroids; LABA – Long-acting beta-agonists; SABA=Short-acting beta-agonists; VAS=Visual analogue scale.
Similar results were observed in sensitivity analyses when considering weeks with at most one day of missing data (e-Tables 6 and 9) or when considering monthly data (e-Table 7).
DiscussionIn this study, there was an overall good adherence to ICS+LABA. Adherence was higher for ICS+other LABA than for ICS+F, but ICS+F was associated with lower SABA use and similar VAS asthma, e-DASTHMA or VAS work levels. Overall, increased adherence to ICS+LABA was associated with decreased SABA use.
As in any mHealth study, there are several limitations to be considered.24 First, there is the possibility of selection biases. MASK-air® users may not be representative of the general population with asthma (being younger and, potentially, with higher access to care). This is exemplified by the fact that, in this study, ICS+F was used more frequently than ICS+other LABA, although this does not occur in many countries. Among MASK-air® users, those who report larger volumes of data may also be different from the remainder. In fact, only 9% of self-reported asthma MASK-air® users (33% of those using ICS-LABA) fulfilled the inclusion criteria (which were set so as to have a sufficiently large continuous period of data collection in order to enable an estimation of medication adherence). This selection may represent another bias since adherence may be lower in weeks with incomplete reporting. However, in a previous study, we found that adherence to the app was not related to adherence to medications.16
Patients were not necessarily enrolled by physicians and we relied on the reported use of asthma medication for identifying patients with asthma. However, in a MASK-air® sub-study of 69 patients, we found that 93% of those with an asthma treatment had a physician diagnosis of current or previous asthma.25 Moreover, ICS+LABA are only used in asthma and chronic obstructive pulmonary disease, with the latter being potentially rare in a sample of patients composed mostly of young adults. Additional limitations include the fact that the severity of asthma, the strength of ICS+LABA and the number of puffs/day were not assessed.
This study also has important strengths. The sample size is quite large, pointing to the possibility of assessing relevant amounts of real-world longitudinal mHealth data from patients with asthma. Additional strengths concern the assessment of (i) periods with no missing data (allowing a full assessment of medication adherence) and (ii) clinically relevant outcomes, such as the use of SABA. Finally, we used data directly provided by the patients, allowing us to overcome information biases in data collection or provision resulting from researchers’ or participants’ expectations about a study.
Even though the use of MASK-air® itself may promote higher medication adherence (currently unclear, to be explored in future studies), the high medication adherence levels found may be largely related to selection biases. In particular, among MASK-air® users, an overrepresentation is expected, not only of younger and more-schooled patients, but also of patients with higher access to specialised health care (even though the app can be found by patients themselves, we estimate that a large amount of them were advised to do so by their physicians). In addition, users highly adherent to the app may be generally more concerned about their asthma and, therefore, more adherent to treatment. This has to be confirmed in new studies. In the assessed patients, medication adherence was higher to ICS+F than to ICS+other LABA. Finding a difference between both medications also suggests that app adherence is unlikely to be the major criterion explaining differences in adherence between different medication schemes. Moreover, this difference was expected, as patients reporting ICS+F may use the medication in the context of the MART approach (MAintenance and Reliever Therapy26) or purely on an on-demand basis.27 Even though this is not possible to assess in MASK-air®, this hypothesis is supported by the fact that ICS+F was associated with a slightly worse control than ICS+other LABA for VAS asthma and e-DASTHMA (although differences were non-meaningful). In the MART approach, patients under ICS+F adapt their treatment depending on symptoms, whereas patients under ICS+other LABA should use regular treatment and SABA when feeling worse.
When patients used ICS+LABA, they were reporting higher VAS asthma and e-DASTHMA levels. This indicates that most patients only use medication when they are not well.
In weeks with SABA use, the percentage of days with SABA differed between ICS+F and ICS+other LABA. This effect was replicated when considering only weeks with adherence. This finding appears to be important, confirming clinical trials and some phase 4 studies with real-life data. Each additional day of ICS+F or ICS+other LABA use is associated with a significant average decrease in weekly SABA use, pointing to the positive impact of a good medication adherence to ICS+LABA.
There were no differences in rhinitis control (CSMS) between ICS+F and ICS+other LABA, supporting the observed results in asthma control.
In conclusion, we compared patients using ICS+F versus ICS+other LABA on their medication adherence. While users under ICS+other LABA displayed higher adherence than those under ICS+F, similar levels of asthma control were observed across the two groups. However, patients under ICS+other LABA displayed a higher frequency of SABA use. An increased medication adherence was found to be associated with lower frequency of SABA use, pointing to the importance of maintaining a high ICS+LABA adherence. Overall, this study shows the potential of mHealth tools in the longitudinal assessment of patients with asthma, allowing physicians and patients to monitor their medication adherence, control and SABA use.
Author contributionsBSP and EMC participated in the study design, data analysis and manuscript writing (original draft); JB and RL participated in the conceptualisation, study design, data analysis, supervision and manuscript writing (original draft); TZ, JMA and JAF participated in the study design, supervision and manuscript writing (revision and editing). All remaining authors participated in the data collection and manuscript writing (revision and editing).
Funding information and role of the sponsorsMASK-air® has been supported by EU grants (POLLAR, EIT Health; Structural and Development Funds, Twinning, EIP on AHA, H2020 and Horizon Europe) and by educational grants from Mylan-Viatris, ALK, GSK, Novartis and Uriach. There was no specific funding for this paper.