It is unclear whether patients with Global Initiative for Chronic Obstructive Lung Disease stage 1 (mild) chronic obstructive pulmonary disease (COPD) have worse respiratory outcomes than individuals with normal spirometry.
MethodsFor this systematic review and meta-analysis, we conducted a search of PubMed, Embase, and Web of Science for all literature published up to 1 March 2023. Studies comparing mortality between mild COPD and normal spirometry were included. A random-effects model was used to estimate the combined effect size and its 95% confidence interval (CI). The primary outcome was all-cause mortality. Respiratory disease-related mortality were examined as secondary outcomes.
ResultsOf 5242 titles identified, 12 publications were included. Patients with mild COPD had a higher risk of all-cause mortality than individuals with normal spirometry (pre-bronchodilator: hazard ratio [HR] = 1.21, 95% CI: 1.11–1.32, I2 = 47.1%; post-bronchodilator: HR = 1.19, 95% CI: 1.02–1.39, I2 = 0.0%). Funnel plots showed a symmetrical distribution of studies and did not suggest publication bias. In jackknife sensitivity analyses, the increased risk of all-cause mortality remained consistent for mild COPD. When the meta-analysis was repeated and one study was omitted each time, the HR and corresponding 95% CI were >1. Patients with mild COPD also had a higher risk of respiratory disease-related mortality (HR = 1.71, 95% CI: 1.03–2.82, I2 = 0.0%).
ConclusionsOur results suggest that mild COPD is associated with increased all-cause mortality and respiratory disease-related mortality compared with normal spirometry. Further research is required to determine whether early intervention and treatment are beneficial in mild COPD.
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration, and/or exacerbations) owing to abnormalities of the airways (bronchitis and bronchiolitis) and/or alveoli (emphysema) that cause persistent and frequent progressive airflow obstruction.1 COPD is one of the leading causes of morbidity and mortality worldwide, and leads to substantial economic, social, and healthcare burdens.2,3 An accurate understanding of the staging and progression of COPD is becoming increasingly important at the individual and population levels, with implications for disease management, population-level prevention and control, and estimation of disease burden.4
Mild COPD is defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.70 and FEV1 ≥80% of the predicted value.1 Patients with mild COPD represent 38%–54% of diagnosed patients with COPD in primary care.5-7 Most patients with mild COPD are asymptomatic and often receive limited or no treatment and insufficient clinical attention.8,9 However, these patients often suffer from considerable morbidity, such as exacerbations, a rapid decline in lung function, limited exercise capacity, and reduced physical activity.9-12 Previous epidemiological studies have examined the relationship between all-cause mortality in patients with mild COPD compared with individuals with normal spirometry, but produced conflicting results. Some studies have shown a higher risk of all-cause mortality in patients with mild COPD than in individuals with normal spirometry.13-16 However, other studies have shown no significant difference in all-cause mortality between patients with mild COPD and individuals with normal spirometry.17-22
Understanding the association between mild COPD and all-cause mortality has important implications for early precautions and managing disease. Therefore, we conducted a systematic review and meta-analysis to evaluate and quantify whether patients with mild COPD have a higher risk of all-cause mortality than individuals with normal spirometry. Moreover, we performed subgroup meta-analyses and generated pooled estimates for smoking status, sex, and follow-up time.
MethodsThis meta-analysis has been registered in the International Prospective Register of Systematic Reviews (registration number: CRD42022360009, www.crd.york.ac.uk/PROSPERO/). This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Literature searchTo identify relevant studies, two investigators (JO and HF) independently searched the Embase, Web of Science, and PubMed databases for studies published from inception to 1 March 2023 with no language restrictions. The search terms and related variants used included “GOLD 1”, “GOLD I”, “GOLD”, “mild COPD”, “mild airflow obstruction”, “mild airflow limitation”, “COPD”, and “mortality”. Further details of the search strategy are shown in Supplement. The terms were chosen by two investigators (JO and HF) and by checking keywords in other articles and reviews on similar topics. References from other related articles and the selected articles were manually reviewed to identify potential studies of interest.
Study selectionTwo investigators (JO and HF) independently reviewed all potentially relevant articles. Disagreements or uncertainties were resolved by a third investigator (FW). The initial investigation included reviews of article titles and abstracts. Articles were mainly excluded because they did not include COPD and mortality. The secondary investigation included a full-text review and selection of articles based on the inclusion and exclusion criteria. The studies included in this systematic review and meta-analysis met the following inclusion criteria: (1) data were available to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality, respiratory-related mortality, cardiovascular disease-related mortality, or cancer-related mortality associated with individuals with mild COPD compared with those with normal spirometry; (2) the studies were independent, and studies with the same data set as published studies were not considered independent; and (3) they were cohort studies. We considered that studies were eligible if they were cohort studies that enrolled adults and reported an association between mild COPD and all-cause mortality. Mild COPD was defined as a pre-bronchodilator FEV1/FVC <0.70 and FEV1 ≥80% of the predicted value.1 Other accepted definitions of mild COPD included a post-bronchodilator FEV1/FVC <0.70 and FEV1 ≥80% of the predicted value. The definitions of normal spirometry included pre-bronchodilator or post-bronchodilator FEV1/FVC ≥0.70 and FEV1 ≥80% of the predicted value. When two studies referred to the same population in the same period and showed overlapping data,19,23 we chose to include articles containing our primary outcome that compared all-cause mortality in patients with mild COPD with that in participants with normal spirometry.19
Data extractionTwo investigators (JO and HF) independently evaluated the quality of all identified studies and extracted and entered the data. These authors then independently verified the quality of the identified studies and the validity of the extracted data. Any disagreements were settled by a third investigator (FW). The following extracted data were recorded: first author, year of publication, location, characteristics of the subjects (sample size, age, diagnostic criteria of mild COPD, and definition of normal spirometry), study design type, and follow-up time.
Quality assessment of included studiesTwo investigators (JO and HF) used the Newcastle–Ottawa quality assessment scale for the quality assessment of cohort studies. In this scale, a study is judged based on selection (four items, one star each), comparability (one item, up to two stars), and exposure/outcome (three items, three stars each).24 The quality of the studies was graded as poor (<4 points), fair (4–6 points), and good (≥7 points).
Summary outcomes and statistical analysisThe primary outcome of this systematic review and meta-analysis was all-cause mortality in patients with mild COPD compared with individuals with normal spirometry. Cardiovascular disease-related mortality, cancer-related mortality, and respiratory disease-related mortality were examined as secondary outcomes. The random-effects model was used to calculate the pooled effect sizes and 95% CI because the studies were conducted over a wide range of settings in different populations. Specific subgroups (sex, different smoking statuses, follow-up time, and different definitions of mild COPD) were examined. We used data on the adjusted outcome in every included study. The I2 statistic was used to evaluate the heterogeneity of the studies. An I2 value of 0%–24% was considered as having no heterogeneity. Greater I2 values represented greater heterogeneity, where I2 values of 25%–49% represented low heterogeneity, 50%–74% represented moderate heterogeneity, and ≥75% represented high heterogeneity.25 The publication bias was evaluated using a funnel plot, Begg's test, and Egger's test.26 All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant. Stata/SE 15.1 (Statacorp LP, College Station, TX, USA) software was used for the meta-analysis.
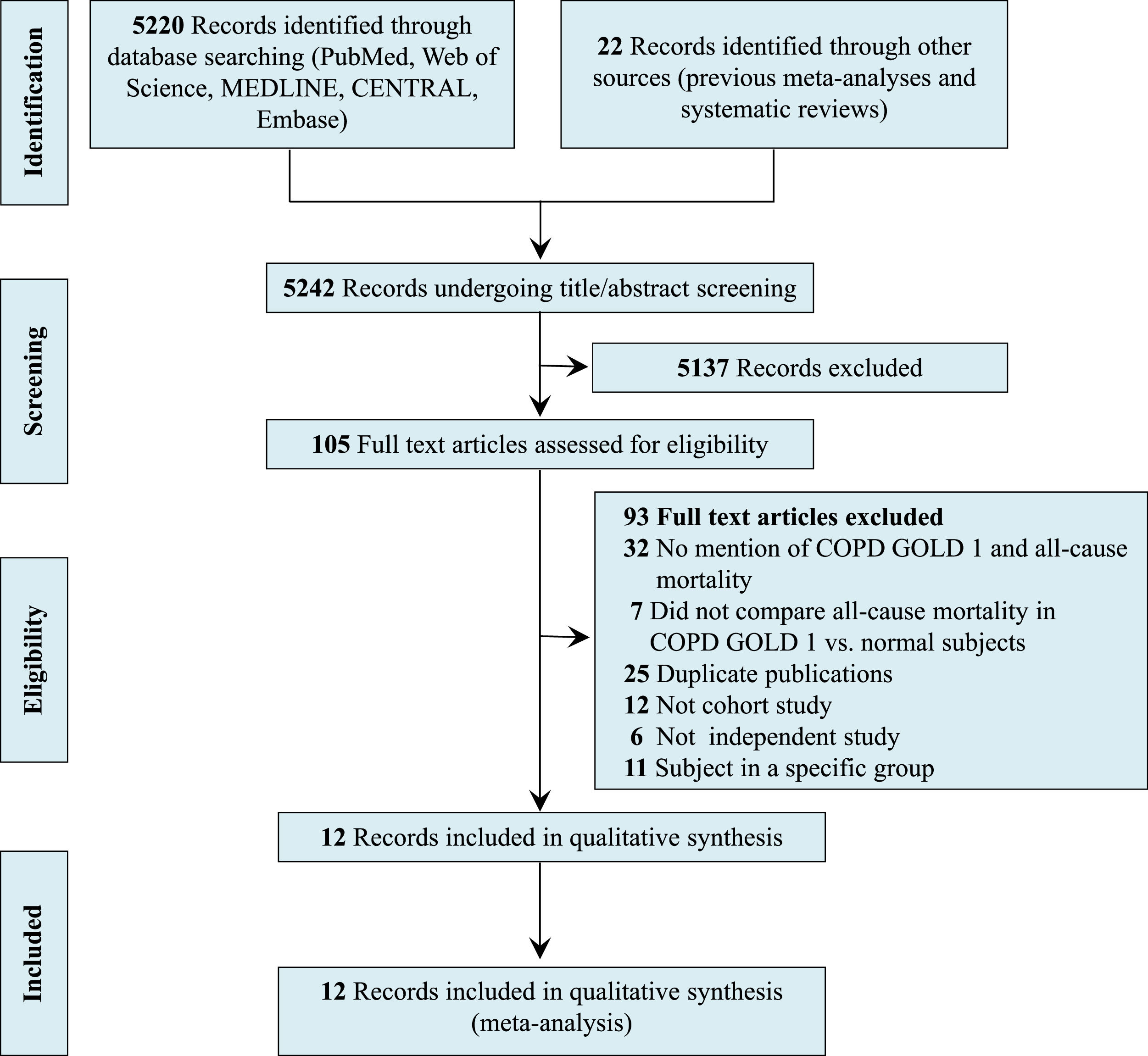
ResultsLiterature searchFig. 1 shows a flow chart of the literature screening, including the key words used in the search strategies, the number of articles identified in the databases, the number of excluded studies, and the reason for exclusion. Of 5,242 abstracts identified during the search, 105 were selected for full-text review and 5,137 were excluded because the topic of this review was not evaluated. After reading the full text, an additional 93 articles were excluded for the following reasons: no mention of mild COPD and mortality, no comparison of mortality in patients with mild COPD versus that in normal subjects, duplicate publications, no cohort study, no independent study, and subjects were in a specific group. Finally, 12 studies were included in the qualitative synthesis (meta-analysis).13-22,27,28
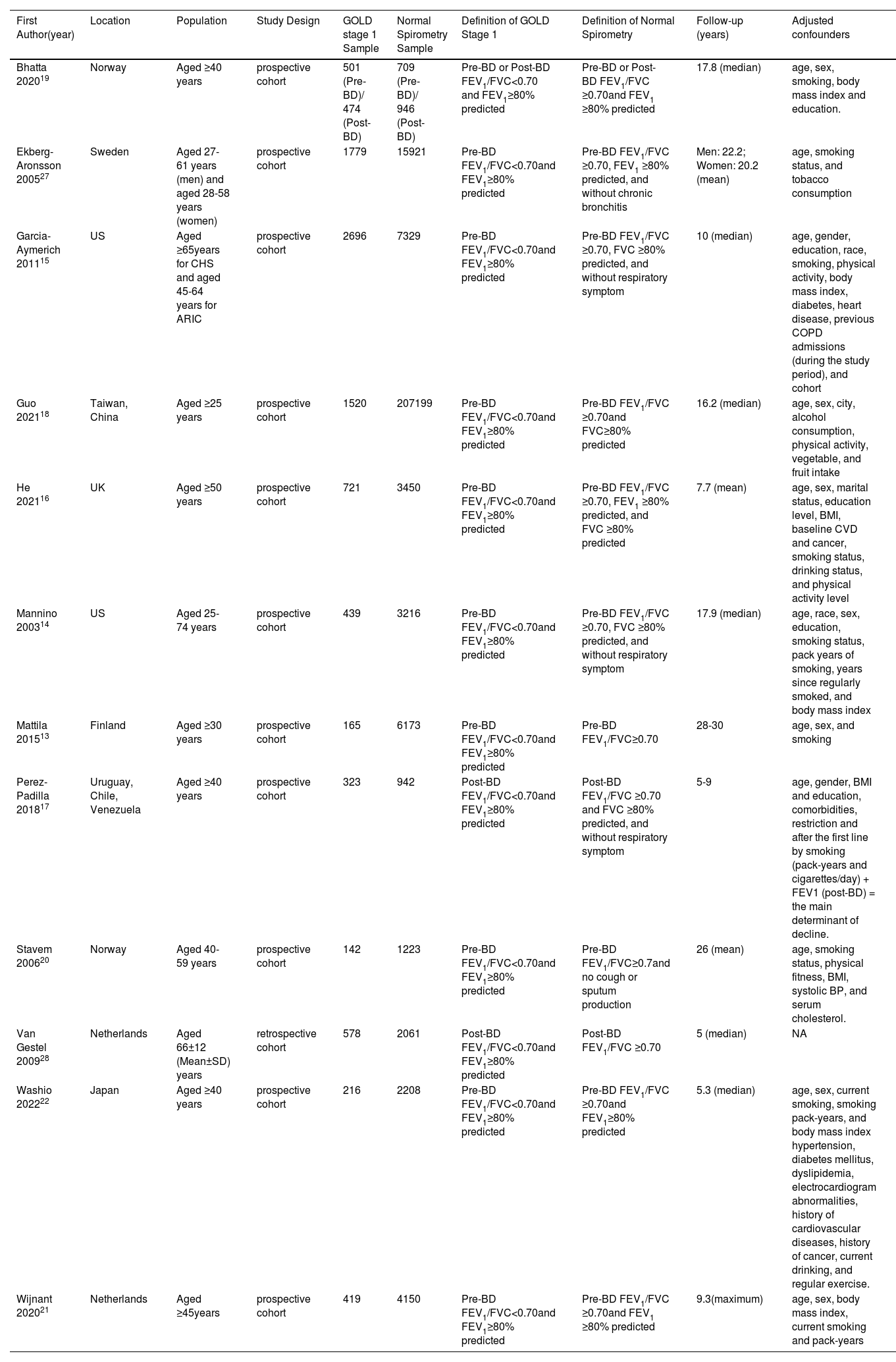
Study characteristicsThe characteristics of the included studies are shown in Table 1. In total, 9,973 participants with mild COPD and 255,527 participants with normal spirometry were included in the 12 studies.13-22,27,28 All data included in our study were adjusted for confounding factors, such as age and body mass index. The average follow-up time of seven studies was ≥10 years,13-15,18-20,27 and the average follow-up time of five studies was <10 years.16,17,21,22,28 Nine studies used pre-bronchodilator FEV1/FVC <0.70 and FEV1 ≥80% predicted as the main definition of mild COPD.13-16,18,20-22,27 Two studies used a post-bronchodilator FEV1/FVC <0.70 and FEV1 ≥80% predicted as the main definition of mild COPD.17,28 One study used both of these definitions for mild COPD.19 All studies were published between 2003 and 2022. The methodological quality of the included studies was satisfactory, with Newcastle-Ottawa Scale 9-point quality assessment scores between 7 and 9. All studies were graded as good quality. The details of the quality assessment are shown in eTable 1 in the Supplementary material.
Characteristics of all studies included in the meta-analysis.
| First Author(year) | Location | Population | Study Design | GOLD stage 1 Sample | Normal Spirometry Sample | Definition of GOLD Stage 1 | Definition of Normal Spirometry | Follow-up (years) | Adjusted confounders |
|---|---|---|---|---|---|---|---|---|---|
| Bhatta 202019 | Norway | Aged ≥40 years | prospective cohort | 501 (Pre-BD)/ 474 (Post-BD) | 709 (Pre-BD)/ 946 (Post-BD) | Pre-BD or Post-BD FEV1/FVC<0.70 and FEV1≥80% predicted | Pre-BD or Post-BD FEV1/FVC ≥0.70and FEV1 ≥80% predicted | 17.8 (median) | age, sex, smoking, body mass index and education. |
| Ekberg-Aronsson 200527 | Sweden | Aged 27-61 years (men) and aged 28-58 years (women) | prospective cohort | 1779 | 15921 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70, FEV1 ≥80% predicted, and without chronic bronchitis | Men: 22.2; Women: 20.2 (mean) | age, smoking status, and tobacco consumption |
| Garcia-Aymerich 201115 | US | Aged ≥65years for CHS and aged 45-64 years for ARIC | prospective cohort | 2696 | 7329 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70, FVC ≥80% predicted, and without respiratory symptom | 10 (median) | age, gender, education, race, smoking, physical activity, body mass index, diabetes, heart disease, previous COPD admissions (during the study period), and cohort |
| Guo 202118 | Taiwan, China | Aged ≥25 years | prospective cohort | 1520 | 207199 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70and FVC≥80% predicted | 16.2 (median) | age, sex, city, alcohol consumption, physical activity, vegetable, and fruit intake |
| He 202116 | UK | Aged ≥50 years | prospective cohort | 721 | 3450 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70, FEV1 ≥80% predicted, and FVC ≥80% predicted | 7.7 (mean) | age, sex, marital status, education level, BMI, baseline CVD and cancer, smoking status, drinking status, and physical activity level |
| Mannino 200314 | US | Aged 25-74 years | prospective cohort | 439 | 3216 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70, FVC ≥80% predicted, and without respiratory symptom | 17.9 (median) | age, race, sex, education, smoking status, pack years of smoking, years since regularly smoked, and body mass index |
| Mattila 201513 | Finland | Aged ≥30 years | prospective cohort | 165 | 6173 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC≥0.70 | 28-30 | age, sex, and smoking |
| Perez-Padilla 201817 | Uruguay, Chile, Venezuela | Aged ≥40 years | prospective cohort | 323 | 942 | Post-BD FEV1/FVC<0.70and FEV1≥80% predicted | Post-BD FEV1/FVC ≥0.70 and FVC ≥80% predicted, and without respiratory symptom | 5-9 | age, gender, BMI and education, comorbidities, restriction and after the first line by smoking (pack-years and cigarettes/day) + FEV1 (post-BD) = the main determinant of decline. |
| Stavem 200620 | Norway | Aged 40-59 years | prospective cohort | 142 | 1223 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC≥0.7and no cough or sputum production | 26 (mean) | age, smoking status, physical fitness, BMI, systolic BP, and serum cholesterol. |
| Van Gestel 200928 | Netherlands | Aged 66±12 (Mean±SD) years | retrospective cohort | 578 | 2061 | Post-BD FEV1/FVC<0.70and FEV1≥80% predicted | Post-BD FEV1/FVC ≥0.70 | 5 (median) | NA |
| Washio 202222 | Japan | Aged ≥40 years | prospective cohort | 216 | 2208 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70and FEV1≥80% predicted | 5.3 (median) | age, sex, current smoking, smoking pack-years, and body mass index hypertension, diabetes mellitus, dyslipidemia, electrocardiogram abnormalities, history of cardiovascular diseases, history of cancer, current drinking, and regular exercise. |
| Wijnant 202021 | Netherlands | Aged ≥45years | prospective cohort | 419 | 4150 | Pre-BD FEV1/FVC<0.70and FEV1≥80% predicted | Pre-BD FEV1/FVC ≥0.70and FEV1 ≥80% predicted | 9.3(maximum) | age, sex, body mass index, current smoking and pack-years |
Abbreviations: BD=bronchodilator; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CHS=the Cardiovascular Health Study; ARIC=the Atherosclerosis Risk in Communities cohort study; SD=standard deviation.
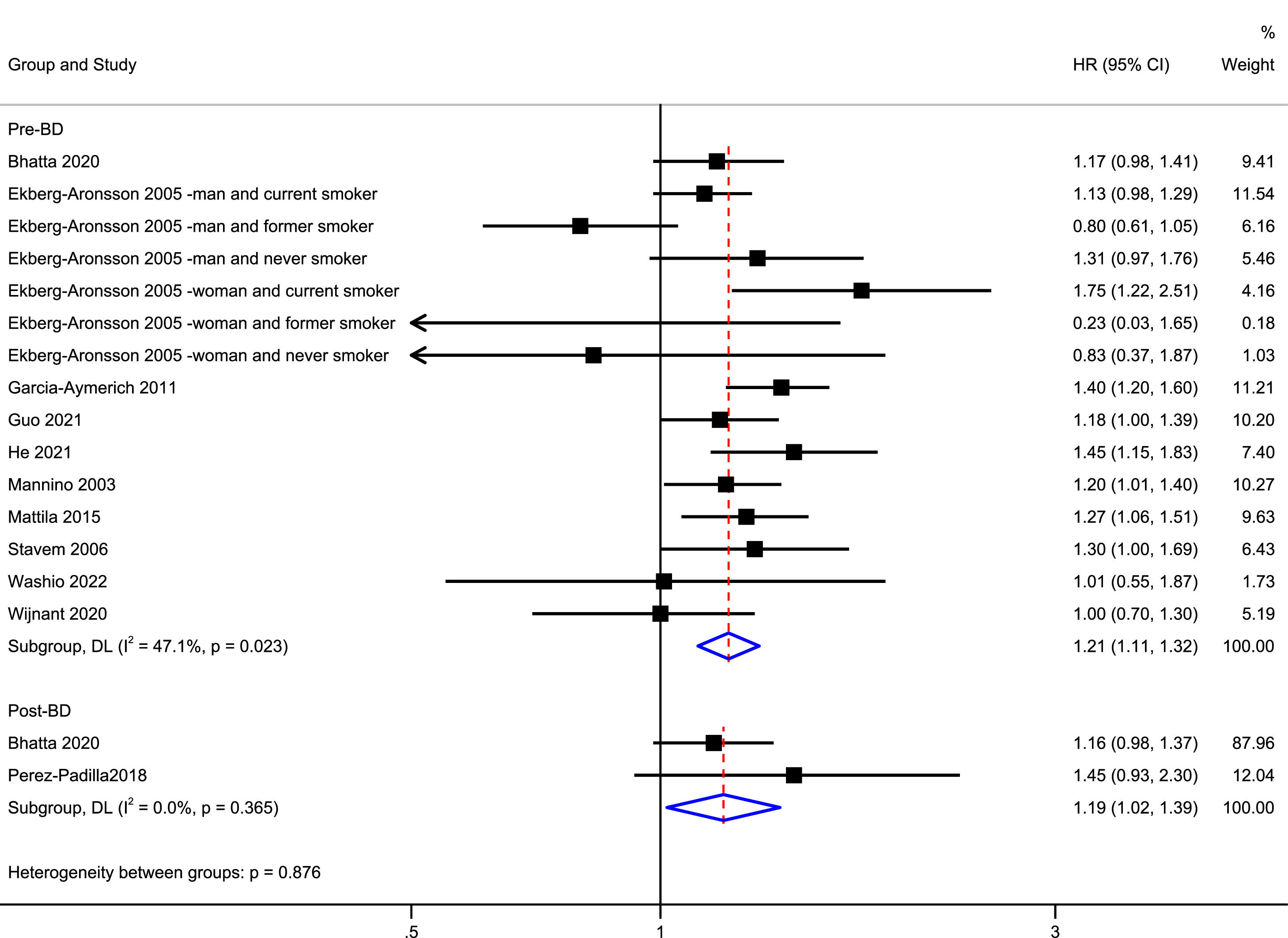
Fig. 2 shows the pooled results for all-cause mortality. Eleven studies examined the association between mild COPD and all-cause mortality.13-22,27 Patients with mild COPD had a higher risk of all-cause mortality than individuals with normal spirometry (pre-bronchodilator: HR = 1.21, 95% CI: 1.11–1.32; post-bronchodilator: HR = 1.19, 95% CI: 1.02–1.39). There was low between-study heterogeneity (pre-bronchodilator: I2 = 47.1%, P = 0.023; post-bronchodilator: I2 = 0.0%, P = 0.365). In jackknife sensitivity analyses, the increased risk of all-cause mortality remained consistent for mild COPD. When the meta-analysis was repeated and one study was omitted each time, the HR and corresponding 95% CI were >1 (Supplementary eFig. 1). Funnel plots showed a symmetrical distribution of the studies and did not show evidence of publication bias (Supplementary eFig. 1). Egger's linear regression (t = −1.13, P = 0.279) showed no obvious publication bias (Supplementary eFig. 1).
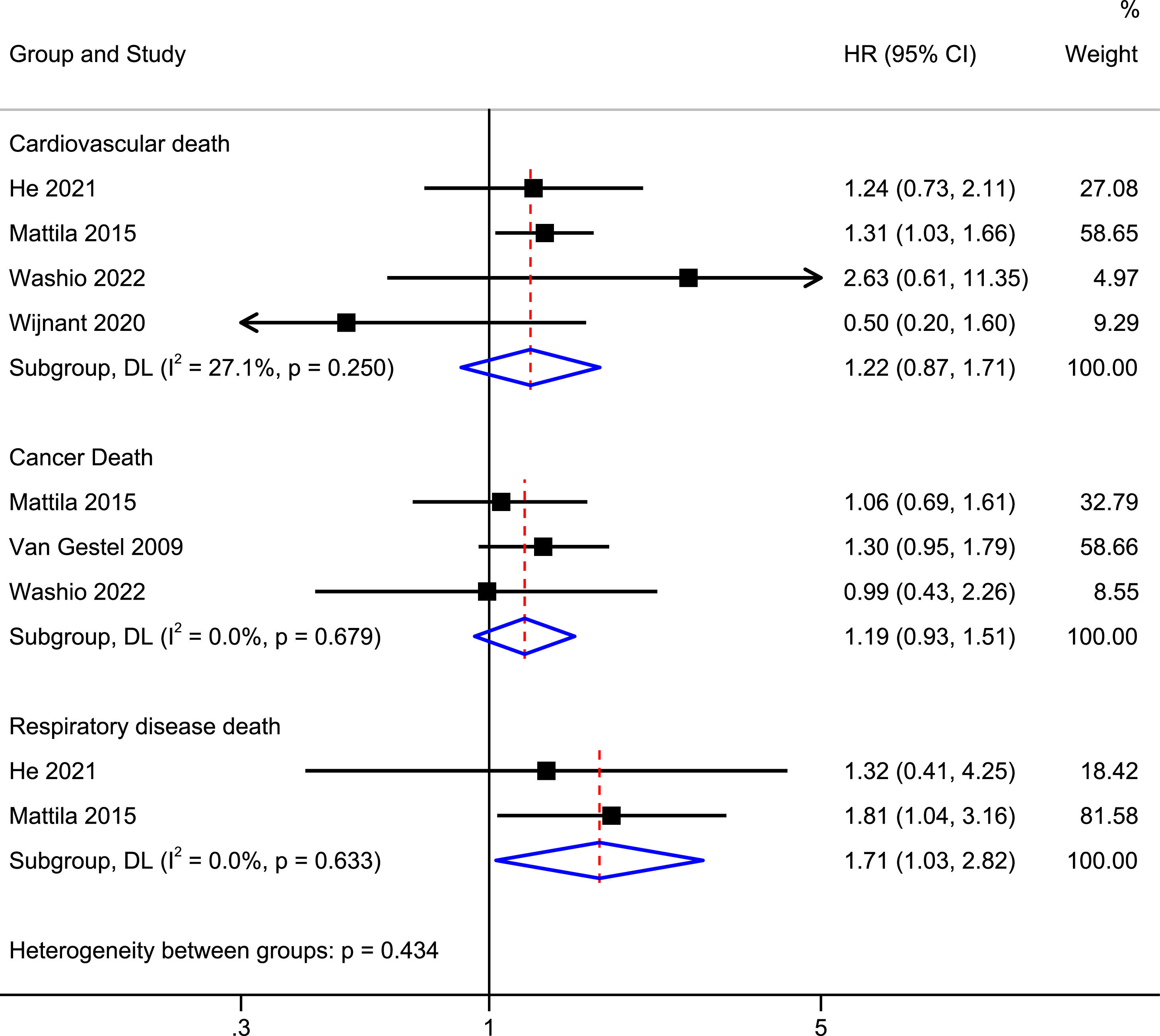
Association of mild COPD with a risk of death from respiratory, cardiovascular, and cancer-related diseasesFig. 3 shows the pooled results of respiratory disease-related mortality, cardiovascular disease-related mortality, and cancer-related disease mortality. Patients with mild COPD had a higher risk of respiratory disease-related mortality than participants with normal spirometry (HR = 1.71, 95% CI: 1.03–2.82, I2 = 0.0%). However, patients with mild COPD did not have a higher risk of cardiovascular disease-related mortality (HR = 1.22, 95% CI: 0.87–1.71, I2 = 27.1%) or cancer-related mortality (HR = 1.19, 95% CI: 0.93–1.51, I2 = 0.0%).
Subgroup analysisSupplementary eTable 2 shows the predefined subgroup analysis of all-cause mortality events for mild COPD. The number of studies in each subgroup was too small to obtain clear research results. The risk of all-cause mortality was higher in patients with mild COPD than in individuals with normal spirometry in current smokers (HR = 1.31, 95% CI: 1.04–1.64, P = 0.021; I² = 62.0%, Tau²=0.0246, P = 0.072) and never smokers (HR = 1.25, 95% CI: 1.08–1.43, P = 0.002; I²=0.0%, Tau²=0.00, P = 0.761). However, the risk of all-cause mortality in patients with mild COPD associated with former smoking was not significant (HR = 0.84, 95% CI: 0.64–1.10, P = 0.205; I² = 15.1%, Tau²=0.0098, P = 0.308). In the follow-up period of ≥10 years, mild COPD was associated with increased mortality (HR = 1.21, 95% CI: 1.10–1.33) and moderate heterogeneity was found (I²=50.8%, Tau²=0.0118, P = 0.022). In the follow-up period of <10 years, mild COPD was not a significant risk factor for all-cause mortality (HR = 1.25, 95% CI: 1.00–1.55; I² = 32.0%, Tau²=0.0158). We also analyzed the HR of all-cause mortality in the two subgroups of male (HR = 1.20, 95% CI: 0.97–1.48, P = 0.094; I² = 75.8%, Tau²=0.0433, P = 0.002) and female sex (HR = 1.17, 95% CI: 0.86–1.57, P = 0.316; I² = 52.4%, Tau²=0.0524, P = 0.078). The subgroup analysis showed no effect of sex on all-cause mortality.
DiscussionThis systematic review and meta-analysis is the first to quantitatively synthesize the current evidence on mild COPD and mortality. We found that patients with mild COPD had higher all-cause mortality and respiratory disease-related mortality than individuals with normal spirometry. This association was present in the subgroup analysis of different definitions of mild COPD.
Previous studies have indicated that, in the mild COPD stage, symptoms have not yet interfered with patients’ daily activities, leading patients and physicians to underestimate the presence of this disease.29 Little research has been performed on mild COPD because patients with this condition do not often seek medical care and are generally excluded from clinical studies.30 However, the actual incidence of exacerbations in patients with COPD and mild airflow limitation may be higher than expected because many exacerbations are likely to be unreported.11 A literature review suggested that patients with mild COPD are at a high risk of disease progression, leading to a substantial disease burden.4 Our study showed that patients with mild COPD had higher all-cause mortality than individuals with normal spirometry. These findings suggest that mild COPD needs to be taken seriously in terms of closer follow-up, appropriate early management, and intervention. Initially, appropriate exercise intervention and follow-up management for patients with mild COPD and deterioration-related risk factors should be performed, followed by pharmacological interventions if progressive exacerbations and deterioration occur.
Some recent studies have shown that exclusive reliance on spirometry in patients with COPD and mild airflow limitation may result in the underestimation of clinically important physiological impairment.31 Patients with mild COPD have measurable physiological impairment with increased morbidity and a higher risk of mortality.30 Pathological abnormalities in the small airways, which include thickening of the airway wall, infiltration of inflammatory immune cells into wall tissue, and occlusion of the small airway lumen by inflammatory mucous exudates, are the major reasons for airway obstruction in patients with COPD and mild airflow limitation.32,33 Reduced pulmonary carbon monoxide uptake capacity, ventilation–perfusion mismatch, an elevated alveolar–arterial oxygen tension gradient, and markedly abnormal pulmonary microvascular blood flow are also components of the pathophysiology of mild COPD.11,32 Quantitative computed tomography scans have shown a broad range of structural abnormalities, such as emphysema, pulmonary gas trapping, airway wall thickening, vascular abnormalities, and bronchiectasis in mild COPD.1,34 Among never-smokers or current smokers, mild COPD was associated with a higher risk of mortality compared with normal spirometry, but this association was not found among those who had quit smoking. This finding could have been due to the small sample size. Additionally, the risk of death in patients with mild COPD who quit smoking was the same as that in individuals with normal spirometry who quit smoking, suggesting a major role of smoking cessation in halting the progression of COPD.
Previous studies and our study did not show a higher risk of cardiovascular disease-related death and cancer death in patients with mild COPD compared with those with normal spirometry.16,21,22,35 This is an unsurprising result because patients with mild COPD have less severe lung structural changes, and less systemic inflammation and cardiovascular disease caused by lung lesions than those with normal spirometry.36
Our study has several strengths. First, to the best of our knowledge, this is the first review to quantitatively compare all-cause mortality in patients with mild COPD with that in individuals with normal spirometry. Second, this review was pre-registered and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement of recommended items, which reduces manipulation and increases transparency. Third, most of the studies that we included were prospective cohort studies. Fourth, we performed meta-analyses using adjusted HRs when determining all-cause mortality in patients with mild COPD. Fifth, the diagnosis of COPD was confirmed by spirometry in all included studies.
LimitationsOur study has several limitations. First, GOLD guidelines recommend the use of post-bronchodilator spirometry to diagnose mild COPD, but most of our included studies used pre-bronchodilator spirometry, and the different diagnostic methods of spirometry may have affected the final assessment of the results. However, previous studies have indicated consistent results in the use of pre- and post-bronchodilator spirometry to diagnose COPD.19 The post-bronchodilator and pre-bronchodilator findings obtained in this study are consistent, supporting the robustness of our results. Second, not all of our pre-defined subgroup analyses on the association of mild COPD with the risk of all-cause mortality showed a positive association. The fact that the number of studies in each subgroup was too small to obtain clear results needs to be taken into consideration. Therefore, further studies on mild COPD and all-cause mortality are still required to assess these subgroups in the future. Third, our analysis was based on pooled data (studies) and not individual data. We had no access to individual participants’ data, and potential confounders could not be ruled out.
ConclusionsThis systematic review and meta-analysis showed that patients with mild COPD have higher all-cause mortality and respiratory disease-related mortality than individuals with normal spirometry. Further research is required to determine whether early pharmacological or non-pharmacological intervention and treatment are beneficial in mild COPD.
Author contributionsDrs Zou, Ou, Wu, Fan, Hou, Li, Deng, Liu contributed to completing the interpretation of the data and the manuscript. Wu, Zou, Ran, Ou, Fan contributed substantially to the concept, design, interpretation of the data, and completion of the study and manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
FundingThis study was supported by the Local Innovative and Research Teams Project of the Guangdong Pearl River Talents Program (2017BT01S155), the Guangzhou Science and Technology Plan Project (202002030080), the Natural Science Foundation of Guangdong Province Project (2020A1515010264), the National Natural Science Foundation of China (81970045, 82270043, and 81970038), the Foundation of Guangzhou National Laboratory (SRPG22-018 and SRPG22-016), the Guangzhou Science and Technology Plan Project (202002030080), and the Natural Science Foundation of Guangdong Province Project (2020A1515010264).
The authors thank all the staff who provided excellent technical support during this study.