Recently there has been growing interest in an alternative to conventional oxygen therapy: the heated, humidified high flow nasal cannula oxygen therapy (HFNC). A number of physiological effects have been described with HFNC: pharyngeal dead space washout, reduction of nasopharyngeal resistance, a positive expiratory pressure effect, an alveolar recruitment, greater humidification, more comfort and better tolerance by the patient, better control of FiO2 and mucociliary clearance. There is limited experience of HFNC in adults. There are no established guidelines or decision-making pathways to guide use of the HFNC therapy for adults. In this article we review the existing evidence of HFNC oxygen therapy in adult patients, its advantages, limitations and the current literature on clinical applications. Further research is required to determine the long-term effect of this therapy and identify the adult patient population to whom it is most beneficial.
Recentemente, uma alternativa à oxigenoterapia convencional tem recebido atenção crescente: trata-se da oxigenoterapia humidificada de alto débito com cânulas nasais (HFNC). Um número de efeitos fisiológicos têm sido descritos: «lavagem» do espaço morto faríngeo, redução da resistência da nasofarige, efeito tipo «CPAP», recrutamento alveolar, maior humidificação, maior conforto e melhor tolerância do doente, melhor controle do FiO2 e do «clearance» mucociliar. A experiência com HFNC em adultos ainda é limitada e de momento não há «guidelines» para o seu uso. Neste artigo revemos a evidência existente do uso da HFNC em adultos, as suas vantagens, limitações e a literatura mais recente sobre as suas aplicações clínicas. Mais investigação será necessária para determinar os efeitos a longo prazo desta terapêutica e identificar quais as populações em que é mais benéfica.
For years supplemental oxygen administration provided by different devices (such as nasal prongs, nose masks and face masks), has been the first line treatment for hypoxemic respiratory failure. However the oxygen provided by these conventional systems has several limitations. These limitations do not usually have clinical consequences because the delivered oxygen flow is sufficient to correct the hypoxemia. However, in some patients there can be serious problems. For example, poor tolerance because of insufficient humidification and heating of the oxygen flow or the fact that the oxygen flow supplied by these devices generally is no more than 15L/min (the maximum flow delivered by facemasks). Another drawback of conventional oxygen devices is the difference between the oxygen flow delivered and that the exact amount of the patient's inspiratory flow is not precise; it can vary between 30 and 120L/min during respiratory failure.1⿿3 This means that the proportion of humidified and oxygenated inspired gas can be very small (below 10%) depending on the extent of oxygen dilution with room air.2 One direct consequence is that the fraction of inspired oxygen (FiO2) is not constant during conventional oxygen therapy and it is also unknown.
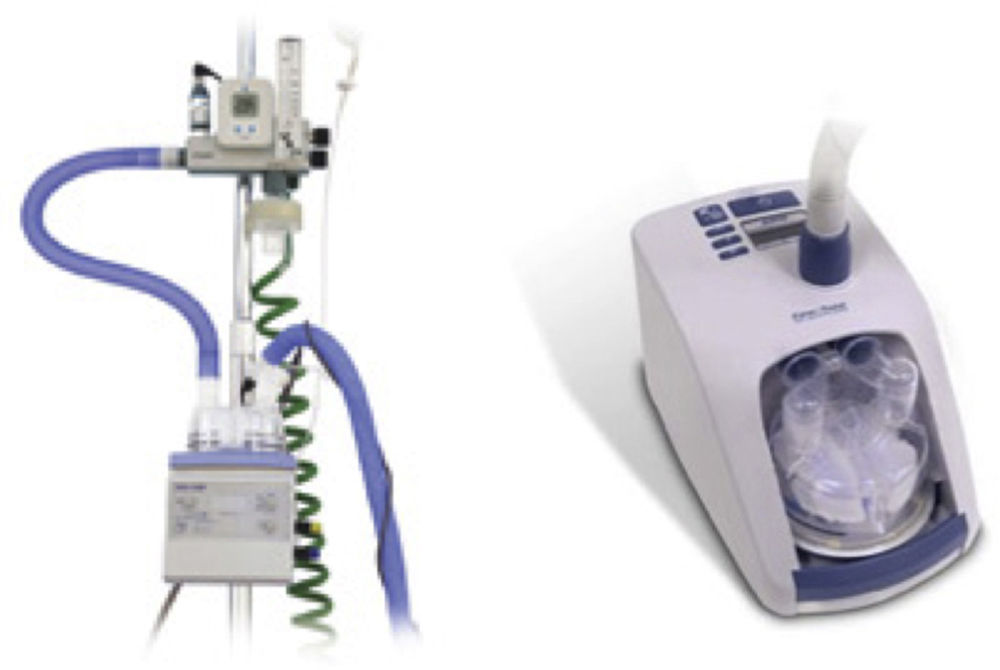
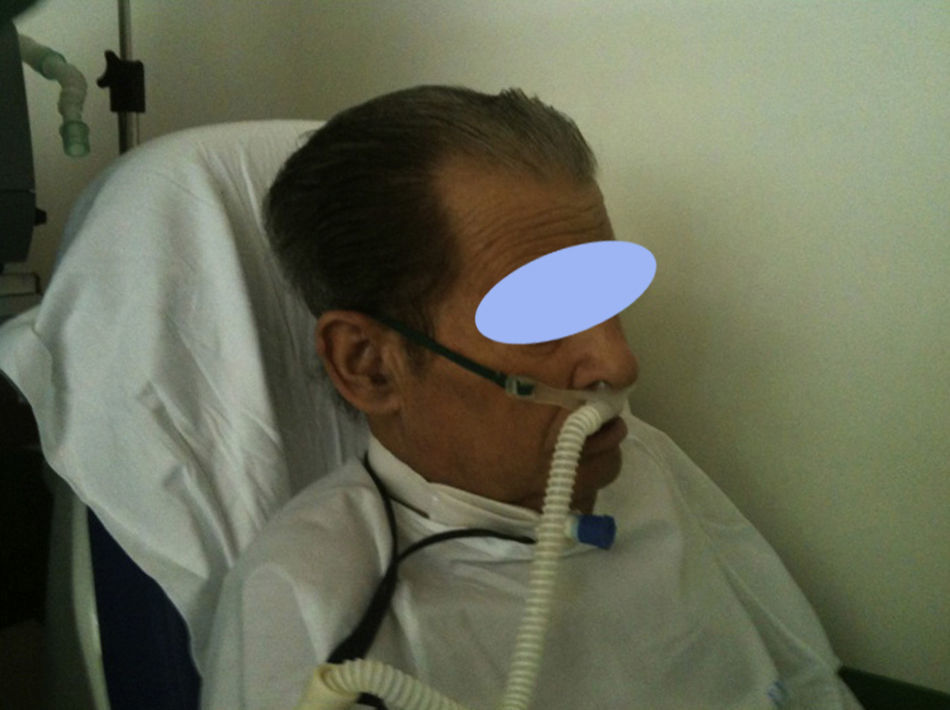
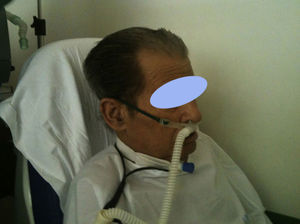
Recently growing attention has been paid to an alternative to conventional oxygen therapy. We refer to the heated, humidified high flow nasal cannula oxygen therapy (HFNC). This system basically works with an air oxygen blender allowing from 21% to 100% FiO2 and generates up to 60L/min flow rates. The gas is heated and humidified through an active heated humidifier and delivered via a single limb heated inspiratory circuit (to avoid heat loss and condensation) to the patient through nasal cannula of large diameter (Figs. 1 and 2), the ⿿high flow nasal cannulas⿿.3 This therapeutic alternative is mainly characterized by the fact that the patient is given a heated, humidified high flow above its maximum inspiratory flow and we can have increased confidence about the real FiO2 being delivered to the patient. HFNC has been widely studied in pediatric patients where it is increasingly used, however, the evidence in adults is limited.4 There are no established guidelines or decision-making pathways to guide use of the HFNC therapy for adults. In this article we review the existing evidence of HFNC oxygen therapy in adult patients, its advantages, limitations and the current literature on clinical applications.
How does HFNC work?HFNC has a number of physiological effects that could be used to illustrate its benefits. Several studies have shown that HFNC generates a low level of positive airway pressure,2,5,6 improves oxygenation, increases the end-inspiratory lung volume, reduces airway resistance, increases functional residual capacity2,7 and flushes nasopharyngeal dead space,2,8 thus helping to manage breathing reduction in acute respiratory failure from all causes. It also better tolerated and more comfortable for the patient. Finally, pulmonary defence mechanisms are restored. The main physiological effects of HFNC are shown in Table 1.
The main effect of delivering high flow oxygen directly into the nasopharynx is to wash CO2 and reduce CO2 rebreathing. This allows the dead space to decrease and increases alveolar ventilation over minute ventilation ratio. These properties have some clinical benefits for exercise tolerance, dyspnea reduction and better oxygenation.
A few years ago, Dewan and Bell,8 studied the clinical impact of high flow oxygen on exercise tolerance and the sensation of dyspnea. For this study, ten COPD patients who were already receiving transtracheal oxygen were recruited. Each subject underwent a total of four modified progressive treadmill tests in a single-blind randomized fashion on two separate days. Two tests were performed with patients receiving low-flow transtracheal oxygen (LFTTO) and high flow transtracheal oxygen (HFTTO), and the other group received low and high flow oxygen by nasal prongs (NP). The flows were adjusted to provide equivalent oxygen saturation in the respective groups. The average distance with HFTTO was 2.5 times greater than with LFTTO, and high-flow NP was 2.38 times higher compared with low-flow NP. Interestingly, there was no significant difference in exercise distance and dyspnea scores with HFTTO as compared with high-flow NP and LFTTO versus low-flow NP. This study shows that the use of high-flow oxygen via both transtracheal catheter and nasal prongs significantly increased exercise tolerance in COPD patients when compared to low-flow oxygen.
The dead space washout also has some beneficial effects in terms of oxygenation as observed by Chatila et al.9 These investigators have conducted a prospective, nonrandomized, nonblinded study aimed at comparing the effects of high flow of humidified oxygen to conventional low-flow oxygen delivery at rest and during exercise in ten patients with COPD. After a period of rest and baseline recordings, patients were asked to exercise on a cycle ergometer for up to 12min. Exercising was started on low flow oxygen first; after another period of rest, the patients repeated exercising using the high-flow oxygen system, set at 20L/min and matched to deliver the same FiO2 as that of low flow oxygen delivery. Patients were able to exercise longer on high flows (10.0±2.4min versus 8.2±4.3min) with less dyspnea, better breathing pattern, and lower arterial pressure compared to low flow delivery. In addition, oxygenation was higher while receiving high flow oxygen at rest and exercise despite the matching of FiO2. The main conclusion of this study was that high flows of oxygen improved exercise performance in patients with COPD and severe oxygen dependency, in part by enhancing oxygenation.
Reduction of nasopharyngeal resistanceAnother described effect is the resistance of the nasopharyngeal air flow. The design of the nasopharynx facilitates humidification and warming of inspired gas by contact with the large surface area. By definition, this large wet surface area and nasopharyngeal gas volume can account for an appreciable resistance to gas flow. In addition, after analyzing nasal and oral flow-volume loops, Shepard and Burger showed10 that the nasopharynx has a distensibility that contributes to a variable resistance. When inspiratory gas is drawn across this large surface area, retraction of the nasopharyngeal boundaries results in a significant increase in inspiratory resistance compared to expiratory resistance. CPAP has been shown to reduce this supraglottic resistance up to 60% by mechanically splinting the airways. However, HFNC most probably minimizes the inspiratory resistance associated with the nasopharynx by providing nasopharyngeal gas flows that match or exceed a patient's peak inspiratory flow. This change in resistance translates into a decrease in resistive work of breathing.3
Positive expiratory pressure (PEEP effect)Physiologically a positive airway pressure effect, generated by high flow oxygen, provides a certain level of pulmonary distending pressure and alveolar recruitment. This effect has been documented in healthy persons by Groves and Tobin.5 In their study the volunteers were fitted with a high flow nasal interface and pharyngeal pressures were recorded with flows from 0 to 60L/min. A flow dependent generation of positive expiratory pressure was measured reaching a median pressure of 7.4cm H2O at 60L/min with the mouth closed. Moreover, they found that expiratory pressures with the mouth closed was higher than with the mouth open and this was statistically significant (<0.001).5 In conclusion, this study shows us that there is a degree of CPAP generated with the HFNC therapy, which is flow dependent and also dependent on whether the person is breathing with mouth open or closed.
These results have been confirmed by Parke et al.11 These authors have studied the relationship between flow and pressure with the HFNC oxygen therapy system. Fifteen patients were invited to participate. These patients were scheduled for elective cardiac surgery. Measurements were performed with nasal high flow oxygen at flows of 30, 40 and 50L/min with the patient's mouth both open and closed, the pressures were recorded over 1min of breathing and average flows were calculated via simple averaging. There was a positive linear relationship between flow and pressure and a mean positive airway pressure of 2.7cm H2O at 35L/min was measured with the mouth closed. A large interpatient variability was also noted by these authors. This variability is probably due to differences in leak around the outer part of the nasal cannula and the wide variability in nare size. A smaller leak may create an increased resistance to expiration resulting in higher nasopharyngeal pressure, i.e., an increase the PEEP effect.6 Although this aspect deserves particular attention in neonates, it could also be potentially useful in adult patients. It might be interesting in adults to minimize leaks around the cannula using cannulas sizes greater than 50% of the nare size.
In conclusion, nasal high flow oxygen is not proposed as an alternative to continuous positive airway pressures or noninvasive ventilation, where controlled pressures are indicated, however, the nasal high flow oxygen might provide a bridge to these therapies in selected patients.11
Alveolar recruitment effectThe high oxygen flows delivered by nasal cannula may correct hypoxemia by several mechanisms and thus contribute to the alleviation of respiratory distress symptoms. The positive airway pressure effect provides a certain level of pulmonary distending pressure and alveolar recruitment, but it is unclear how their use affects lung volume.12 A recent study by Corley et al.13 assessed twenty patients prescribed HFNC post-cardiac surgery. Electrical lung impedance tomography was used to assess changes in lung volume by measuring changes in lung impedance. The primary objectives were to investigate the effects of HFNC on airway pressure, end-expiratory lung volume and to identify any correlation between the two. Authors measured a significant correlation between end-expiratory lung impedance and airway pressure compared with low-flow; the high flow nasal cannula significantly increased end-expiratory lung impedance and airway pressure. Tidal impedance was also increased with HFNC. The authors also found that these results were most beneficial in patients with higher body mass indexes. In conclusion this study is important because it shows that at least a part of the improvement in oxygenation observed in patients with acute respiratory failure (ARF) is due to alveolar recruitment.
Humidification and toleranceGiven some of the reported issues associated with a conventional oxygen mask and breathing dry gas and cold air (mask discomfort, nasal dryness, oral dryness, eye irritation, nasal and eye trauma, gastric distension and aspiration) nasal high flow oxygen may have an important role11 and the need to heat and humidify supplemental oxygen has been long debated.2 In comparison to high flow face mask oxygen, some studies have found better comfort, tolerance and oxygenation and lower respiratory rate with HFNC.1,11,14 Chanques et al.,15 in a study including 30 patients treated by high-flow oxygen therapy showed that bubble humidifiers delivered poor levels of humidity and were associated with significant discomfort; however the use of a heated-humidifier in patients with high-flow oxygen therapy was associated with a decrease of dryness symptoms mediated by increased humidity levels. Because high flows of cold and dry oxygen used during HFNC therapy increase the airway resistance, the addition of heat and humidity are compulsory with HFNC.3,16
The heated humidifier system may also indirectly affect oxygenation. Active humidification improves mucociliary function, facilitates secretion clearance and decrease atelectasis formation which improves the ventilation-perfusion ratio and oxygenation.1 In a recent study by Sztrymf et al.12 about the impact of high-flow nasal cannula oxygen on intensive care unit patients with acute respiratory failure, there were no reported interruptions of HFNC therapy because of discomfort. In another study in which the HFNC was used for an average of 2.8±1.8 days (maximum 7 days), intolerance never caused HFNC to be discontinued and no unexpected side effects were reported.2 Therefore, we believe that HFNC could be considered very comfortable, especially because all the patients chose to continue with HFNC.1
Better control of FiO2 and better mucociliary clearanceIn addition, other proposed mechanisms of action may include the ability to more accurately control the patient's FiO211,14,17 and better mucociliary clearance.11,18
Primary mechanical pulmonary defence mechanisms are sneezing, coughing, gagging and the use of natural filters, i.e., nasal hairs. The second line of defence is the mucociliary transport system which traps and neutralizes inhaled contaminants (in mucus) and transports them up and out of the airway, keeping the lung free from infection-causing pathogens. This critical defence system is very sensitive to humidity. Loss of humidity can be a problem in itself.
In the clinical settings there are several situations where moisture is reduced. It is the case of delivering gas from an artificial flow source, such as piped oxygen, or the utilization of an endotracheal or tracheostomy tube bypassing the upper airway where the majority of humidification would naturally occur. These factors deplete the airway mucosa of heat and moisture and this can have significant adverse effects on the function of the mucociliary transport system and lead to impaired airway defence and gas exchange: 1. The mucus layer becoming thick and tenacious; 2. The thickness of the aqueous layer decreasing, causing cilia to slow down or stop; and 3. Heat loss from the epithelium cells, making cilia beat less frequently.19
Delivering essential humidity through HFNC can prevent drying of the airway, avoiding the inflammatory response caused by the drying of the mucosa. Conditioning of the gas can also minimize airway constriction, reducing the work of breathing, which helps to maintain effective delivery of oxygen to the lungs. By delivering optimal humidity, patients can maintain the function of the mucociliary transport system, clearing secretions more effectively and reducing the risk of respiratory infection. This can be particularly important for patients with secretion problems such as those with COPD. All these beneficial effects are directly related to humidification hence the name of active humidification.20
Clinical evidence of HFNCThe HFNC has a good profile in terms of clinical and physiological parameters such as dead-space washout, nasopharyngeal resistance reduction, positive pharyngeal pressure, alveolar recruitment, oxygen dilution reduction and enhanced mucociliary function.3 These beneficial effects led the researchers to assess this therapy in the clinical setting. The main areas in which evidence is available are shown in Table 2.
There is limited published experience with HFNC in adults with ARF. Roca et al.1 were the first to present promising data on respiratory and oxygenation parameters in twenty patients in the intensive care unit (ICU). They showed significant improvement in both clinical and physiological parameters after 30min of HFNC in comparison with standard facemask oxygen therapy. It is worth noting that the median duration of conventional treatment before starting HFNC was more than 4 days, which precludes any conclusion on the effect of HFNC in the immediate management of ARF. In addition, HFNC was only used for 30min, so no data can be obtained about the long-term effects of this device.
Recently, a first experience with high-flow nasal cannula therapy has been reported by Sztrymf et al.12 in twenty patients with persistent acute hypoxemic respiratory failure despite oxygen with conventional facemask and without indication for immediate intubation. The etiology of ARF was mainly pneumonia (n=11), sepsis (n=3) and miscellaneous (n=6). These patients had a moderate to severe respiratory failure with a median respiratory rate of 28bpm and a median pulse oxymetry of 93.5% under a median of 15L/min oxygen with a facemask. The use of HFNC enabled a significant reduction of respiratory rate to a median of 24.5 breath per minute (p=0.006) and a concomitant significant increase in oxygen saturation to 98.5% (p=0.0003). The HFNC was well tolerated with a median duration of 25.5h and a maximum of 156h. In this small series, six patients ultimately required intubation, providing a 70% success rate for the technique. The same authors have confirmed these results in a larger cohort of patients and have identified early predictors for HFNC failure.2 In fact, a persistence of tachypnea, thoraco-abdominal asynchrony and lower pulse oxymetry were significantly more frequent in patients ultimately requiring intubation. In conclusion these studies have shown for the first time the beneficial effects of HFNC in patients with ARF. Their main results can be summarized as follows: (1) all respiratory parameters were improved after 1h of HFNC, achieving a rapid alleviation of respiratory distress in more severe patients; (2) use of HFNC led to a significant improvement in oxygenation; (3) HFNC was well tolerated for long periods (a maximum of 7 days) with sustained benefits in patients who were not intubated; and (4) the success rate of this technique was high (70%).
In less severe patients, HFCN was compared to facemask oxygen therapy in a prospective randomized comparative study conducted by Parke et al.17 Investigators analyzed the success with the allocated therapy and subsequent need for non invasive ventilation as principal outcomes in 60 patients with mild to moderate hypoxemic respiratory failure. In this study, significantly more HFNC patients succeeded with their allocated therapy and the rate of non invasive ventilation was 3 out of 29 patients with HFNC (10%) and 8 out of 27 patients with facemask oxygen (30%). Patients with HFNC also had significantly fewer desaturations. According to these authors, HFNC may be more effective than high flow facemask in treating mild to moderate hypoxemic respiratory failure.
Finally, in a recent study with thirty-eight patients with respiratory failure caused by community-acquired pneumonia, the use of HFNC was associated with a significant reduction in respiratory rate, heart rate, dyspnea score, supraclavicular retraction, thoracoabdominal asynchrony and significant improvement in a pulse oxymetry. These improvements were seen as early as 15⿿30min after the beginning of HFNC and 6h after for heart rate. The PaO2 was significantly higher after 1h than before use of the device. The PaO2/FiO2 ratio was significantly improved at 1 and 24h when compared with the value observed before use of HFNC, however there was no significant increase in pH and PaCO2 before, after 1 and 24h of HFNC on arterial blood gases.2
These results obtained in the ⿿real life⿿ of the management of ARF indicate that patients can be safely managed for several days with HFNC. This technique offers an effective alternative to conventional oxygenation.12 Despite these promising results, controlled studies are needed to assess whether HFNC reduces intubation or not.
Postextubation periodHigh-flow oxygen therapy is a routine treatment for hypoxemic respiratory failure in self extubated patients in the ICU. This therapy is traditionally delivered via a face mask rather than nasal cannula, because of the flow limits of traditional nasal cannula and the tendency for patients in respiratory distress to breathe through their mouths.17 Recently with the introduction of the HFNC, due to their beneficial effects and better tolerance demonstrated in several studies1⿿3,11,12 it seems worth investigating its use either to prevent or to treat postextubation respiratory failure.3 There is limited available literature about this topic. In an Italian study,21 109 patients were randomized to receive either venturi facemask oxygen or high-flow nasal cannula oxygen. All analyzed parameters (respiratory rate, oxygenation, device displacement, comfort) favored the use of HFNC. Reintubation was significantly less frequent in the HFNC group (3.5%) that in the venturi mask group (21%) although one may argue that this latter figure seems unusually high. Nonetheless, this study clearly shows the potential benefit of this technique in improving comfort and enhancing oxygenation in the postextubation period.3 In another recent study Tiruvoipati et al.,14 compared the efficacy of high-flow nasal cannula oxygen and high-flow face mask (HFFM) in fifty extubated patients. All the patients were randomized to either protocol A (HFFM followed by HFNC) or protocol B (HFNC followed by HFFM) after a stabilization period of 30min after extubation. The HFNC proved an effective therapy delivering oxygen to extubated patients who require high-flow oxygen and the tolerance of HFNC was significantly better than in HFFM (p=0.01).
The use of HFNC in the decannulation process of tracheostomized patients in the context of difficult weaning, is another promising area of development (Fig. 3) but there are no publications about this yet (just personal experience).
PreintubationEndotracheal intubation in critically ill patients is associated with severe life-threatening complications in about 20% mainly due to hypoxemia.22 Non-invasive ventilation can be used to enhance oxygenation before tracheal intubation,23 but the mask has to be removed during the laryngoscopy which deprives the patient of oxygen during the procedure. In these cases the nasal cannulas do not interfere with the laryngoscopy and HFNC could be used to deliver oxygen during the apneic period of tracheal intubation.3 A recent experimental study in eight anesthetized piglets with collapse-prone lungs induced by lung lavage, showed that direct pharyngeal administration of 10L/min oxygen during intubation, delayed the time to severe desaturation during apnea, suggesting that this technique might be useful when intubating critically ill patients with acute respiratory failure.22 The potential benefit of HFNC during intubation should be further evaluated in a clinical study. However, for ethical reasons, the design of the study cannot be that of a randomized controlled trial.3 It would not be ethical to perform a randomized controlled study comparing HFNC and conventional face mask oxygen because the amount of published data clearly shows the superiority of HFNC.
The main question that remains without a definitive answer is whether or not HFNC reduces the need for intubation in patients with hypoxemic acute respiratory failure. Some clinicians have the impression that in some instances, use of HFNC has avoided intubation; this has not yet been shown in a controlled trial. However there are some indications in the literature that it may do so. A recent study which evaluated the clinical impact of HFNC in patients with severe respiratory failure found a success rate of 68%,2 i.e., only 32% of patients treated with HFNC required subsequent mechanical ventilation (invasive or non-invasive). However we will have to wait for the results of the FLORALI study, a randomized controlled trial that compares three methods: conventional oxygen therapy, HFNC and HFNC with noninvasive ventilation.3
Use of HFNC in the emergency departmentDyspnea and hypoxemia are one of the most common complaints in patients who come to the emergency department (ED) and oxygen therapy is one of the first treatments provided, according to current guidelines. It can be delivered by face mask or nasal prongs depending on the severity of the patient's respiratory distress. Rapid relief of the dyspnea and correction of hypoxemia are not always achieved by conventional oxygen,3 to which must be added the limited amount of oxygen supplied, the considerable imprecision regarding exactly how much FiO2 was delivered and the poor tolerance of oxygen by some patients because of insufficient heating and humidification.24 The potential benefit and feasibility of HFNC in ED was recently evaluated by a prospective, observational study in a university hospital emergency department.24 Seventeen patients with acute respiratory failure requiring >9L/min oxygen or ongoing clinical signs of respiratory distress despite oxygen therapy were studied. The patients were treated with HFNC after having received conventional oxygen therapy via a facemask. The dyspnea rate by the Borg scale and a visual analogue scale (VAS), respiratory rate (RR) and pulse oxymetry (SpO2) were collected before and 15, 30, 60min after beginning HFNC. This new device was associated with a significant decrease in both dyspnea score (Borg scale from 6 to 3 [p<0.001] and VAS from 7 to 3 [p<0.01]), RR decreased from 28 to 25 (p<0.001) and SpO2 increased from 90% to 97% (p<0.001). HFNC enabled a rapid and significant improvement of dyspnea score and other parameters. HFNC was also well tolerated, more comfortable and no more difficult to use than conventional oxygen therapy via a facemask. These results suggest that HFNC could constitute a first line therapy for selected patients coming to the ED with ARF.24 However, more studies are required to show whether or not early application of HFNC avoids ICU admission in patients presenting to the ED with ARF.3
Bronchoscopy and others invasive proceduresDuring bronchoscopy gas exchange is usually impaired owing to sedation and mismatching of the ventilation relationship.25 Hypoxemia is common with this technique because the PaO2 usually drops approximately 20mmHg during the procedure and the worst decrease occurs during bronchoalveolar lavage (BAL).25⿿29 Age, gender and baseline peripheral oxygen saturation (SpO2) are not reliable predictive variables of hypoxemia25,30 which may persist several hours after the procedure25,31 and increase the incidence of cardiac arrhythmia.25,32 To avoid bronchoscopy-induced hypoxemia, oxygen supply can be delivered by interfaces fed with low or high gas flow. An alternative method that has been successfully used is noninvasive ventilation during bronchoscopy procedures in high risk patients. A randomized study has recently been published which includes forty-five patients receiving oxygen therapy during bronchoscopy [40L/min through a venturi mask (V40), nasal cannula (N40) and 60L/min through a nasal cannula (N60)]. The duration of the procedure was similar in all groups as well as the midazolam used (4mg in each group). Gas exchange and circulatory variables were sampled before (FiO2=0.21) at the end of bronchoscopy (FiO2=0.5) and thereafter (V40; FiO2=0.35). At the end of bronchoscopy HFNC with 60L/min (N60) presented higher PaO2, PaO2/FiO2 and SpO2 than N40 and V40 which were both the same. In conclusion under a flow rate of 40L/min both the venturi mask and HFNC behaved in a similar way, but nasal cannula associated with a 60L/min flow produced better results, thus supporting its use in mild respiratory involvement.25,33 Our group has also recently performed a randomized pilot study comparing conventional oxygen administration with HFNC during fiberoptic bronchoscopy in mildly hyoxemic patients which showed a better level of comfort with the latter.34 Before HFNC can be recommended in this setting more studies with a wider population and more severely hypoxemic patients are needed.
The HFNC may also be used in other invasive procedures such as transoesophageal echocardiography or digestive tract endoscopy when performed in hypoxemic spontaneously breathing patients.3
Palliative careRespiratory signs and symptoms can be distressing for patients, families, caregivers and physicians who care for cancer patients35 and patients with advanced respiratory disorders. Physicians are ethically obligated to recognize, evaluate and consider the best treatment for dyspnea.36 Supplemental oxygen represents one such treatment modality and it is widely utilized in institutional settings as well as at home.37 Possible benefits include symptomatic and functional improvement, as well as the perception that oxygen is life-sustaining. Patients with underlying hypoxia are more likely to benefit,38 however, in certain settings there is no significant dyspnea reduction between hypoxic and non-hypoxic patients.39 Two randomized double-blind cross-over studies40,41 comparing air versus oxygen in cancer patients with dyspnea, as well as a consecutive cohort study42 and a metaanalysis43 in dyspneic patients, all failed to demonstrated a symptom benefit even when oxygen saturation improved. Most recently, a randomized controlled double-blind multinational trial of oxygen versus room air, both via nasal cannula, in 239 outpatients with refractory dyspnea demonstrated no significant differences in the palliation of breathlessness.44 Recently, a study performed at Memorial Sloan Kettering Cancer Center35 including 183 medical records patients, analyzed the utilization of humidified high-flow nasal oxygen in oncological patients with dyspnea. These patients had a variety of malignancies including: hematological (29%); lung (17%); gastrointestinal (15%); sarcoma (6%), head, neck and central nervous system (5%), breast (4%) and other tumors (24%). The majority of patients were administrated HFNC for hypoxia (98%; including 37 postoperative or post-procedure patients) and had underlying cardiopulmonary disease (93%; including contributing thromboembolic and neurologic disease). HFNC was used in the ICU in 72% of cases and also in the hospital ward alone or after an ICU stay. The patients treated with the HFNC usually improved (41%) or remained stable (44%), while 15% deteriorated. These patients have been treated over the past two years and the device generally seemed well tolerated.35 Additionally, HFNC was effective in the stabilization or improvement of respiratory difficulties in the majority of treated patients, often obviating the need for ICU admission or for invasive ventilatory treatments such as mechanical ventilation. At study completion, 45% of patients were living and 55% had died. The median time of use of HFNC was 3 days. There was a do-not-resuscitate (DNR) order for 101 (55%) patients, either before or after device utilization.33,35
The strengths of this analysis include the fact that this sizable cohort constitutes, as far as we know, the only clinical description of the HFNC device exclusively in the cancer population. It can be concluded that the HFNC is safe and well tolerated, with no potential risk beyond those associated with traditional oxygenation strategies (e.g., flammability). Among patients and health cares providers (physicians, nurses and respiratory therapists) the most common anecdotal benefit of HFNC compared with devices allowing equivalent amounts of oxygen delivery is that users are still able to eat and talk unencumbered.35 This technique provides acceptable conditions to manage respiratory failure in palliative patients.3
In our experience, HFNC also has significantly improved oxygenation and cough compared with non-rebreather oxygen mask in severely hypoxemic end-stage patients with interstitial lung disorders, allowing for a better interaction with the families.36 While the practice might be debated, patients with respiratory failure who have declared that they do not want to be intubated or resuscitated are commonly treated with non-invasive ventilation (NIV) and could potentially benefit from HFNC.
Recently, Peters et al.45 identified fifty do-not-intubate (DNI) and do-not-resuscitate (DNR) patients with hypoxemic and mild hypercapnic respiratory distress who were admitted to the ICU and who received HFNC before proceeding to NIV. Patient diagnoses were pulmonary fibrosis (15), pneumonia (15), Chronic Obstructive Pulmonary Disease/COPD (12), cancer (7), hematologic malignancy (7) and congestive heart failure/CHF (3). The HFNC therapy was initiated at a mean FiO2 of 0.67 and flow rate 42.6L/min. Mean O2 saturations went from 89.1% to 94.7% (p<0.001) and respiratory rate 30.6⿿24.7 per minute (p<0.001). It is worth noting, however, despite the overall illness severity, only 18% of patients progressed to NIV, while 82% were maintained on HFNC with a median duration of 30h. This study was observational but this topic should be studied prospectively.
Acute heart failureIt is common to find patients with acute heart failure (AHF) who, after being stabilized, maintain a level of dyspnea or hypoxemia which does not improve with conventional oxygenation systems. One study by Carratalá Perales et al.46 has been recently published including five patients with AHF due to acute pulmonary edema (APE) and refractory hypoxemia at 24h after admission. All patients were treated with conventional oxygen systems in a short stay unit and non-invasive ventilation in the emergency room (3 patients with constant positive airway pressure and 2 with a bi-level pressure device) and afterwards with HFNC. The clinical, arterial blood gas parameters and the degree of the dyspnea were improved in all patients and improvement was observed after 24h of treatment with HFNC system (significant reduction in the intensity of the dyspnea, improved respiratory effort and tachypnea and disappearance of hypoxemia). The improvement with this system may have two main causes: first, this device provides a more constant FiO2 and second, the use of a nasal cannula as the interface reduces the amount of respiratory dead space and generates a constant positive pressure directly proportional to the flow used and the resistance created during expiration, which contributes to increased oxygenation.46,47 In short, the use of HFNC is a good alternative to traditional oxygenation systems for the treatment of patients with ARF secondary to AHF due to APE that have dyspnea and refractory hypoxemia.46 It is characterized by easy administration and management, general perception of improved patients tolerance/comfort with minimal nasal trauma, and patients outcomes are similar to those described with CPAP use.
Chronic airway disordersChronic obstructive pulmonary disease (COPD) and bronchiectasis are both airway disorders characterized by neutrophilic airway inflammation, mucus hypersecretion and retention, and impaired mucociliary transport.48⿿53 A number of treatment strategies to improve mucociliary clearance have been employed. These include physical methods54 and mucoactive drugs which have been shown to improve mucus clearance and health related quality of life (QOL).55 Hasani et al.56 demonstrated that as few as 3h/day of humidification therapy over seven days for bronchiectasis patients significantly increased lung mucociliary clearance measured by radioaerosol labeling. Mall et al.57 demonstrated in a mouse model that airway surface dehydration leads to persistent neutrophilic airway inflammation with increased mucus production and resultant emphysema. Taken together, these studies suggest that airway surface dehydration may play an important role in the pulmonary damage associated with chronic airway disorders. However, the effects of long-term humidification therapy (LTHT) in patients with chronic airway disorders are currently unknown. Recently Rea et al.48 have presented a 12-month randomized study with 108 patients diagnosed with COPD or bronchiectasis with daily humidification therapy. The aim of this study was to examine the effects of LTHT on frequency of exacerbations, QOL, lung function, exercise capacity and airway inflammation. A clinical diagnosis of COPD was confirmed with spirometry and defined as an FEV1 of less than 70% of predicted, an FEV1/FVC ratio <70% without significant bronchodilatador reversibility. Bronchiectasis was confirmed by high-resolution computed tomography (HRCT). Patients with bronchiectasis associated with cystic fibrosis or hypogammaglobulinemia were excluded. The results show that the patients on long-term humidification therapy had significantly fewer exacerbation days (18.2 versus 33.5 days; p=0.045), increased time to first exacerbation (median 52 versus 27 days; p=0.0495) and reduced exacerbation frequency (2.97/patient/year versus 3.63/days/patient/year; p=0.067) compared with usual care. Quality of life scores and lung function improved significantly with humidification therapy compared with usual care at 3 and 12 months. In conclusion, this data demonstrated that averaging as little as 1⿿2h/day of LTHT significantly decreases all the parameters included in this study.48
We have had the opportunity to verify the effectiveness of the HFNC in a severe COPD patient suffering from chronic cough which interfered with sleep and was exacerbated by the administration of O2. This opens new areas of research in the field of HOT, while identifying the need to individualize the prescription of oxygen therapy.58
Others uses of HFNCThe experience with HFNC oxygen therapy in severe acute respiratory infection (SARI) is limited. One study was described by Rello and colleagues in adult patients with SARI confirmed 2009 influenzae/H1N1v infection (by real-time reverse transcription polymerase chain reaction testing).59 The high-flow nasal cannula was indicated in the presence of acute respiratory failure when the patient was unable to maintain a pulse oxymetry of more than 92% with more than 9L/min of oxygen using a standard face mask conventional delivery systems. Nonresponders were defined by their need for subsequent mechanical ventilation. Twenty-five nonintubated adult patients were admitted for SARI (21 pneumonia). Twenty were unable to maintain pulse oxymetry of more than 92% with conventional oxygen administration and required HFNC oxygen therapy, which was successful in 9 (45%). All 8 patients on vasopressors required intubation within 24h. After 6h of HFNC oxygen therapy, nonresponders presented a lower PaO2/FiO2 (median, 135 [interquartile range, 84⿿210] versus 73 [56⿿61] mmHg p<.05) and needed a higher oxygen flow rate. No secondary infections were reported in health care workers. No nosocomial pneumonia occurred during HFNC oxygen therapy. These results show that therapy with this device appears to be an innovative and effective modality for early treatment of SARI patients, but we still need more studies to demonstrate its effectiveness in this context.59
Technical issuesHFNC devices require 3 components: a patient interface, a gas delivery device to control flow and FiO2, and a humidifier.
Patient interfaceSeveral manufacturers provide cannulas with standard dimension prongs which are designed for high-flow applications. These cannulas can accommodate a high inlet flow of at least 60L/min. The Fisher & Paykel Optiflow cannulas use a different design. The nasal prongs are held in place on the upper lip with an elastic over-ear head band. There is a larger diameter flex tubing proximal to the prongs and an around-the-neck elastic that connects to support the weight of the connecting tube. There also are adapters for tracheostomized patients (Fig. 4).
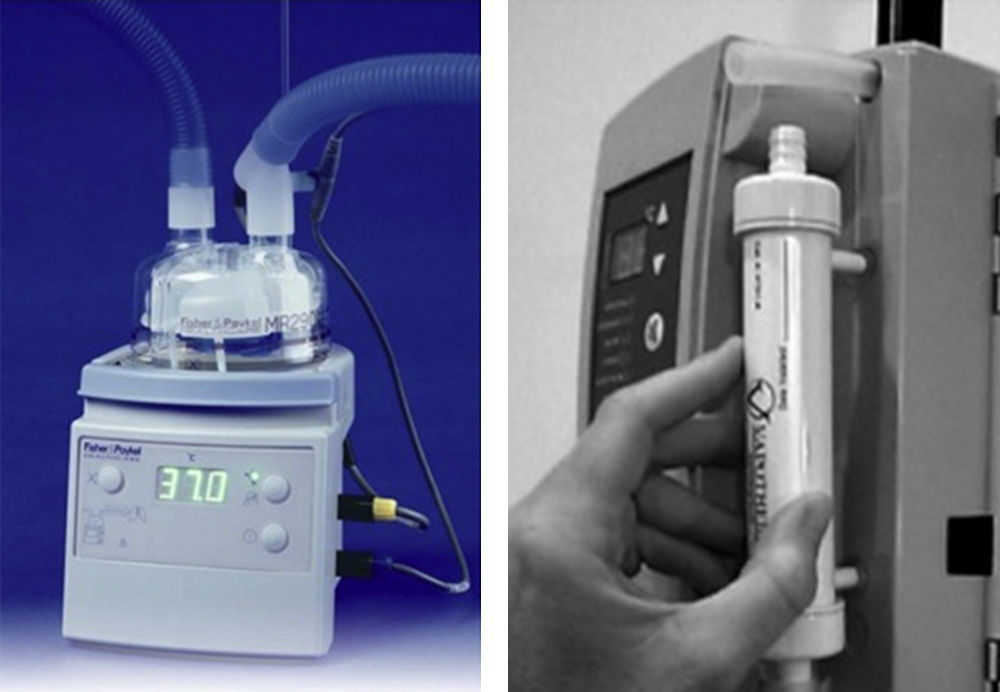
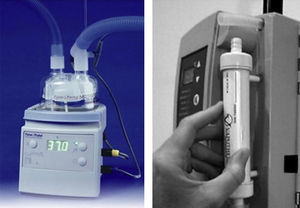
Controlling flow and FiO2We need to use commercially available calibrated high-flow (0⿿70L/min) oxygen flow meters. To allow independent adjustment of FiO2 less than 1.0, separate high-flow air and oxygen flow meters can be connected via a ⿿Ypiece⿿ adapter. High-flow air/O2 proportioner valve blenders or high-flow ⿿Venturi⿿ air mixing valves can be used. In any case, an oxygen analyzer is needed to confirm the FiO2 is appropriate (Fig. 5).
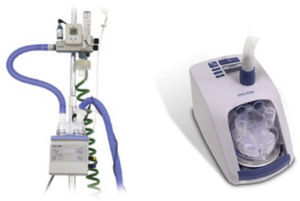
HumidifiersA key element for clinical use of HFNC is effective humidification. The two most popular commercial HFNC devices are the Fisher & Paykel Optiflow and the Vapotherm Precision Flow HFNC. They have different characteristics and technology development (Fig. 6).
The Fisher & Paykel Optiflow HFNC became commercially available in 2006. The system uses a heated humidifier with hot-plate and single-use water chamber, similar to those for application for noninvasive or invasive mechanical ventilation. Humidified gas mixtures exit the humidifier through large bore corrugated tubing that connects to the cannula with a 15mm outer diameter adapter. A heated-wire circuit is used to minimize condensation to prevent liquid water from potentially obstructing the HFNC.
Vapotherm technology is different from the conventional heated plate humidifier systems. This device incorporates a patented vapor transfer cartridge system that allows water vapor to diffuse into the respiratory gas stream while heating the gases to the prescribed temperature (typically 37°C). This system is fundamentally different from the conventional heated plate humidifier systems. The Vapotherm device also employs a triple lumen ⿿jacketed⿿ delivery tube and proprietary nasal cannula optimized to maintain temperature and to minimize condensation (rainout). These latter two features protect the state of respiratory gases so that the gas reaches the patient at the same temperature and humidification state that was achieved in the membrane cartridge. In 2008 Vapotherm released its Precision Flow high-flow humidification system where the air/O2 blender and oxygen analyzer are integrated within the humidifier module.
Both Optiflow and Vapotherm have developed simpler devices designed for home and hospital ward (Figs. 1 and 2). The AIRVO 2 (Fisher & Paykel) sets a new standard for delivering Optiflow to patients, providing performance and convenience with its integrated flow generator and innovative oxygen delivery system. This device is able to deliver close to 100% relative humidity at body temperature (37°C), dew point temperature display and precise, convenient FiO2 delivery from 21% to 80%. It is easy to set up and use with simple controls, integrated O2 mixing, inbuilt O2 sensor and no probes or external air supply required.
Flowrest® is Vapotherm's high flow therapy device designed specifically for homecare and other low acuity environments. Designed with ease of use in mind, the Flowrest® is an integrated system for delivering warmed, humidified breathing gases via a simple nasal cannula. The device has an integrated flow generator, so no external air source is required.
Set up of equipmentHFNC is a very easy treatment to implement. Select appropriate size nasal cannula and circuit tubing for patient size. After this, connect nasal cannula to adaptor on circuit tubing, and connect circuit tubing to humidifier. Attach air and oxygen outlets and connect oxygen tubing from blender to humidifier. Attach water bag to humidifier and turn on to 37°C. The water bag must run freely and be placed as high as possible above the humidifier to achieve flow of water into the humidifier chamber. The system is then ready for use. These steps can vary depending on whether we use Optiflow or Vapotherm devices, a conventional heated plate humidifier systems or a vapor transfer cartridge system.
Check the prongs sit well into the nares. Prongs should not totally occlude nares. Set the high flow nasal cannula system starting with low flows up to prescribed flows. Start off at 6L/min and increase up to goal flow rate over a few minutes to allow patient to adjust to high flow. Flows of 2L per kg per minute with a maximum flow of 60L/min are recommended. As a starting point use 35L/min. Select the FiO2 to obtain the desired arterial oxygen saturation. Because flows used are high, heated water humidification is necessary to avoid drying of respiratory secretions and to maintain nasal cilia function. Set humidifier on the desired temperature. In the acute setting close patient monitoring is necessary, specially respiratory rate, heart rate, degree of chest in-drawing, work of breathing and arterial oxygen saturation and even arterial blood gases where indicated. Within 2h it should be possible to reduce the FiO2 and clinical stabilization should be observed. When oxygen is reduced until a FiO2 of 40% decrease the flow in 5L/min decrements. The evolution of clinical and physiological parameters should allow us to establish the time for step down to conventional oxygen and weaning of HFNC.
ConclusionHFNC has been used for years in neonates with good results, but there is little information about treating ARF adults with these devices. We think that the HFNC could be used as an intermediate therapy to improve oxygenation in adult critical care patients, respiratory care units and also for palliative care. However, we believe that further research is required to determine the long-term effect of this therapy and identify the adult patients population to whom it is most beneficial.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestJoão Carlos Winck has received speaking honoraria from Fisher & Paykel Healthcare in the past three years.