The human congenital central hypoventilation syndrome (CCHS) is caused by mutations in the PHOX2B (paired-like homeobox 2B) gene. Genetically engineered PHOX2B rodents exhibit defective development of the brainstem retrotrapezoid nucleus (RTN), a carbon dioxide sensitive structure that critically controls expiratory muscle recruitment. This has been linked to a blunted exercise ventilatory response. Whether this can be extrapolated to human CCHS is unknown and represents the objective of this study.
Materials and methodsThirteen adult CCHS patients and 13 healthy participants performed an incremental symptom-limited cycle cardiopulmonary exercise test. Responses were analyzed using guideline approaches (ventilation V'E, tidal volume VT, breathing frequency, oxygen consumption, carbon dioxide production) complemented by a breathing pattern analysis (i.e. expiratory and inspiratory reserve volume, ERV and IRV).
ResultsA ventilatory response occurred in both study groups, as follows: V'E and VT increased in CCHS patients until 40 W and then decreased, which was not observed in the healthy participants (p<0.001). In the latter, exercise-related ERV and IRV decreases attested to concomitant expiratory and inspiratory recruitment. In the CCHS patients, inspiratory recruitment occurred but there was no evidence of expiratory recruitment (absence of any ERV decrease, p<0.001).
ConclusionsAssuming a similar organization of respiratory rhythmogenesis in humans and rodents, the lack of exercise-related expiratory recruitment observed in our CCHS patients is compatible with a PHOX2B-related defect of a neural structure that would be analogous to the rodents' RTN. Provided corroboration, ERV recruitment could serve as a physiological outcome in studies aiming at correcting breathing control in CCHS.
Animal studies have shown that rhythmic breathing depends on two coupled medullary oscillators1 (Fig. 1A and 1B). The preBötzinger Complex (preBötC) generates inspiration and the parafacial respiratory group (pFRG) drives active expiration.2,3 The retrotrapezoid nucleus (RTN), a cluster of neurons adjacent to pFRG, plays a crucial role in carbon dioxide sensitivity4–6 and is involved in adapting alveolar ventilation to increased carbon dioxide expiration during exercise.7 Experimentally reducing RTN activity decreases the tidal volume (VT) responses to both hypercapnia and exercise.3,8 Of major pathophysiological significance, the RTN is almost or completely absent in rodents bearing mutations of the paired-like homeobox 2B (PHOX2B) gene.4–6
Summary of the study hypothesis. Panel A. Anatomical organization of the brainstem respiratory network in humans (left and top), inferred from analogies with rodent data and human data (see Schwarzacher, S. W., U. Rub and T. Deller 2011, reference 39 in the manuscript for the putative localization of the human pre-Bötzinger complex (preBötC); see Levy, J., F. Droz-Bartholet, M. Achour, P. Facchinetti, B. Parratte and F. Giuliano 2021, reference 15 in the manuscript for the putative location of the retrotrapezoid nucleus [RTN]). The image shows the localisation of the hypoglossal motor nucleus (XII), the nucleus ambiguus, the facial motor nucleus (VII), the inferior olive (IO), the lateral reticular nucleus (LRN), the parafacial respiratory group (pFRG) which contains the ventral parafacial nucleus (pFV, originally and still most often referred to as the RTN) and the lateral parafacial nucleus (pFL), and the ventral respiratory column (VRC) which contains the preBötC. The Fig. follows the hypothesis that the preBötC mostly drives inspiratory muscles (diaphragm and external intercostal muscles, shown in red) and that the pFRG mostly drives expiratory muscles (internal intercostal and abdominal muscles, shown in blue). Panel B. Conceptual representation of the organisation of respiratory central pattern generators, with inspiratory-related circuits shown in red and expiratory-related circuits shown in blue. The rostral ventral respiratory group (rVRG) contains inspiratory neurons and premotor neurons. The caudal ventral respiratory group (cVRG) contains expiratory premotor neurons. This organisation mainly involves three essential components: 1) an (inexorable) inspiratory oscillator in preBötC that drives inspiration by exciting inspiratory premotor neuronal populations, e.g., rVRG and parahypoglossal region (XII), and inhibits pFL; 2) a (conditional) expiratory oscillator in pFL that gates and drives expiration by exciting expiratory premotor neuronal populations, such as cVRG and XII, and assures alteration of phases by exciting neurons that inhibit preBötC, e.g., inhibitory neurons in either the preBötC or BötC; and 3) a source of tonic drive in pFV (RTN) which derives from dorsal progenitor cells that express paired-like homeobox 2B (PHOX2B) that are able to detect signals related to CO2 and/or pH levels (possibly with the involvement of glia) and integrates other sensory afferents affecting respiratory drive, via excitatory connections to preBötC, BötC, and respiratory premotor neurons, such as rVRG, cVRG, and XII. Respiratory muscles are activated at a certain frequency (FB) and intensity to generate tidal volume (VT). The excitation of rVRG activates inspiratory muscles that produce the inspired component of tidal volume (VTi), at rest and its exercise-related increases. The excitation of cVRG activates expiratory muscles that produce the expired component of tidal volume (VTe), particularly during exercise. Panel C. Tidal volume (VT) dynamics at rest and during exercise in healthy humans (“controls”, top) and patients with congenital central hypoventilation syndrome (“CCHS”, bottom). At rest, inspiration is active and expiration is passive, breathing therefore occurring above functional residual capacity (FRC) defined as the respiratory system relaxation volume (dotted horizontal line). An inspiratory reserve volume (IRV) separates the end of tidal inspiration from full inspiration, and an expiratory reserve volume (ERV) separates the end of tidal expiration (i.e. FRC) from full expiration. The human ventilatory response to exercise is primarily achieved through an increase in tidal volume (VT) that encroaches on both IRV and ERV through the recruitment of inspiratory and expiratory muscles, as depicted on the top trace. Assuming that the human organisation of respiratory rhythmogenesis is similar to that described in rodents, we reasoned that PHOX2B-related RTN/pFRG dysfunction in CCHS patients would result in defective exercise-induced expiratory recruitment. The figure is original and based on a drawing commissioned to and contributed by artist Pierre Bourcier (Paris, France).
In humans, such mutations are responsible for congenital central hypoventilation syndrome (CCHS),9 formerly referred to as Ondine's curse syndrome. CCHS patients suffer from defective breathing control, with life-threatening sleep-related alveolar hypoventilation requiring ventilatory assistance at night and during naps.10 These patients have absent or blunted responses to hypercapnia and hypoxia.10–13 They also have diminished ventilatory response to exercise.14 Although a possible human homologue of the RTN has recently been identified,15 it is currently impossible to link human PHOX2B mutations and functional respiratory perturbations to RTN abnormalities.
Bearing in mind that in the human ventilatory response to exercise VT increases through the recruitment of both inspiratory muscle to take advantage of the inspiratory reserve volume (IRV) and expiratory muscle to exploit the expiratory reserve volume (ERV) (Fig. 1C),16 and assuming that the human organisation of respiratory control is similar to that described in rodents, we reasoned that PHOX2B-related RTN dysfunction in CCHS patients would result in defective exercise-induced expiratory recruitment. To test this hypothesis (summarised in Fig. 1), we conducted a detailed analysis of the ventilatory response to exercise in adult CCHS patients and compared it to the corresponding response in healthy controls.
MethodsSetting and participantsCCHS patientsWe studied 13 non-smoking, clinically stable adult patients with CCHS (Table 1). The study was conducted at the adult branch of the French national reference center for CCHS, located in a 1600-bed tertiary university hospital (Pitié-Salpêtrière, Paris, France). Inclusion criteria were: CCHS diagnosis with documented PHOX2B mutations; age above 18 years; being routinely followed-up at the Pitié-Salpêtrière national adult CCHS reference center (yearly complete workup performed over a single week). Non-inclusion criteria were: pregnancy; body mass index (BMI) >35 kg.m2); restrictive or obstructive ventilatory defects on lung function testing; any contra-indication to exercise testing. Data were obtained prospectively as part of the routine follow-up of the patients at the reference center. The research was carried out in accordance with the principles of the Declaration of Helsinki. The study received the approval of the Institutional Review Board of the French society for respiratory medicine (Comité d'évaluation des protocoles de recherche observationnelle, Société de pneumologie de langue française, CEPRO 2018–016) and patients consented to the use of their clinical data for research purposes.
CCHS and healthy subjects’ characteristics and resting lung function.
Results are presented as median (IQR 25 %−75 %) and as percentage (%) of predicted normal values. CCHS: congenital central hypoventilation syndrome; BMI: body mass index; PARM : polyalanine repeat expansion mutation; NPARM : non polyalanine repeat expansion mutation; FEV1: forced expiratory volume in one second; VC max: maximal vital capacity; TLC: total lung capacity; FRC: functional residual capacity; RV: residual volume; IC: inspiratory capacity; ERV: expiratory reserve volume; PaO2, PaCO2: partial pressure of oxygen/carbon dioxide in arterial blood, respectively; SaO2: arterial oxygen saturation; /: not performed. Please note that there were no statistical differences between CCHS subjects and healthy controls in terms of sex, age, height, weight, BMI and lung function test variables. Blood gases while breathing room air at rest and the ventilatory response to CO2 were not available for healthy controls as they were selected from a healthy-control database used in previous publications (references 17 and 18 in the main manuscript) in which blood gases and the ventilatory response to CO2 were not performed. Please also consider that PHOX2B testing was only available in CCHS patients, for the same reasons described above.
A control dataset was constituted by selecting healthy subjects from a database used in previous publications17,18 using the exact same cardiopulmonary exercise testing (CPET, see below) methodology as in the CCHS patients. The resulting cohort comprised 13 lifelong non-smoking, healthy individuals with normal pulmonary function, matched with the CCHS cohort for age, sex, and BMI.
Descriptive dataIn the CCHS patients, anthropometric data were collated from patients’ medical charts, together with genetic data, the complete description of CCHS-related manifestations and treatments, and medical history. Lung function tests were performed using Medisoft (Belgium) and SentrySuite (Germany) according to the current recommendations of the European Respiratory Society,19,20 and expressed as absolute value and as percentage of predicted value.21 Blood gases while breathing room air at rest were analysed using Radiometer ABL and Siemens Rapidlab blood gas analysers. The ventilatory response to CO2 was assessed using Read's re-breathing method.22 In the controls, only resting lung function tests and exercise variables (see below) were available.
Exercise testingSymptom-limited incremental CPET was conducted on an electrically braked cycle ergometer (COSMED Bike, Cosmed, for CCHS patients and Ergometrics 800S, SensorMedics for controls) with a CPET system (Cosmed, Rome, Italy for CCHS patients and Vmax29c, SensorMedics for controls) as previously described.23,24 All exercise tests consisted of a steady-state resting period of 6 min and a 3-minute warm-up of unloaded pedaling followed by an incremental test in which the work rate (WR) was increased in 1-minute intervals by increments of 5–15 W for CCHS patients and 20 W for controls until the point of symptom-limitation (peak exercise). Participants were instructed to maintain the pedaling rate between 50 and 70 rpm.
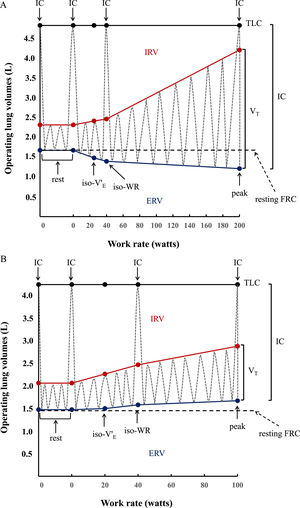
Inspiratory capacity (IC) maneuvers (maximum inhalations starting from end expiration) were performed at rest, at every second minute during exercise, and at peak exercise.23,24 Inspiratory reserve volume (IRV) was calculated as the difference between IC and VT (IRV=IC-VT). Expiratory reserve volume (ERV) was calculated as the difference between the resting forced vital capacity (FVC) measured immediately before starting CPET and IC (ERV=FVC-IC) (Fig. 2A and 2B), based on total lung capacity (TLC) and FVC not changing significantly during exercise.25 Tidal flow-volume curves were constructed at rest, every 2 min during exercise and at peak exercise, and plotted within their respective maximal flow-volume envelopes according to coinciding IC measurements.
Operating lung volume (in liters, L) plot versus cycle work rate (WR, in watts). Inspiratory capacity maneuvers (dotted grey lines) and tidal volume evolution (dotted gray lines) are shown at rest (0 W), iso-ventilation (iso-V'E), iso-work rate (iso-WR) and at peak exercise. TLC = total lung capacity; IRV = inspiratory reserve volume (shown in red); ERV = expiratory reserve volume (shown in blue); VT = tidal volume; IC = inspiratory capacity. Data are presented as means of the whole group in healthy controls (2A) vs means of the whole group in congenital central hypoventilation syndrome (CCHS) patients (2B). In the controls, VT increases with work rate by encroaching on both IRV and ERV, whereas in CCHS patients ERV is not recruited so that VT increases by encroaching on IRV. Dot points indicating IC show the IC manoeuver performed at rest, at iso-WR and at peak during the exercise testing.
Breath-by-breath data were collected while subjects breathed through a facemask with attached low-resistance flow transducer: oxygen consumption (V'O2), carbon dioxide production (V'CO2), ventilation (V'E), tidal volume (VT), breathing frequency (fB), end-tidal oxygen and carbon dioxide partial pressure (PETO2 and PETCO2, respectively) were measured or calculated. Electrocardiographic monitoring of heart rate (HR), rhythm, ST-segment changes, blood pressure (by indirect sphygmomanometry) and oxygen saturation (by ear lobe sensor pulse oximetry [SpO2]), were carried out continuously at rest and throughout exercise testing. In CCHS patients only, measurements of arterial partial pressure of oxygen and carbon dioxide (PaO2 and PaCO2, Torr) were obtained at rest and at peak exercise. Exercise variables were measured continuously and averaged over the last 20 s of each minute and at peak exercise. Verbal encouragement was given during the tests, which could be interrupted either by the patient in the presence of intolerable symptoms or by the operator in the presence of indications to stop exercise.26
Peak work rate (WR peak), peak V'O2 (V'O2 peak) and peak V’E (V’E peak) were defined, respectively, as the highest level of exercise and the highest V'O2 and V’E that could be sustained for at least 30 s during the last stage of exercise. V'O2 peak was reported in absolute units, after correction for body weight, and as a percentage of predicted normal values accounting for age, weight, and sex.27 Breathing pattern during exercise was evaluated by individually plotting VT as a function of V’E (VT/V’E relations or Hey plots).28 Inspiratory flow reserve, defined as the distance between tidal inspiratory flows produced during exercise and those obtained during maximal volitional effort at rest during an FVC maneuver (an indirect index of inspiratory muscle constraint/fatigue), was assessed using the technique described by Johnson et al.29 The presence or absence of expiratory flow limitation was determined by comparing tidal expiratory flows with those of the maximal envelope at isovolume.29 The highest equivalent and common WR and V’E achieved by each participant (healthy controls and CCHS subjects) during all the tests was used as iso-WR and iso-V’E respectively to compare responses at identical physiological stimuli during standard CPET. Metabolic and ventilatory responses at iso-V'E and at iso-WR were calculated by linear interpolation between adjacent measurement points for each subject. Iso-WR and iso-V’E points were calculated to counteract the effect that interindividual WR and V’E may have on the variability of the ventilatory breathing pattern and respiratory mechanics (IRV and ERV) response to a standardized stimulus (WR or V’E) during exercise.
Statistical analysesData were expressed as median and 25th-75th quartile range. Comparisons were performed using the Mann-Whitney U test. CCHS patients and controls were compared at rest, at common standardized exercise WR (40 W: iso-WR), at common standardized V’E (15 L·min−1: iso-V’E) and at peak exercise. All statistical procedures were carried out using Intercooled Stata 8.0 for Windows (Stata, College Station, TX, USA) and SPSS 18.0 for Windows (SPSS Inc, Chicago, IL, USA). Differences were considered significant when the probability P of a type I error was <0.05.
ResultsParticipants’ characteristics and resting lung function testing are summarised in Table 1. There were no statistical differences between CCHS subjects and healthy controls in terms of sex, age, height, weight, BMI and lung function test variables (Table 1). Blood gases while breathing room air at rest and the ventilatory response to CO2 were not available for healthy controls as they were selected from a healthy-control database used in previous publications17,18 in which blood gases and the ventilatory response to CO2 were not performed. For the same reasons described above, the PHOX2B testing was not available in historical healthy subject cohort.
Our study included a genotypically and phenotypically homogeneous set of CCHS patients: twelve CCHS patients presented with PHOX2B polyalanine repeat expansion mutation (PARM) while only one CCHS patient presented with non-polyalanine repeat expansion mutation (NPARM). Among patients with PARM (n = 12): four presented with 5 additional Alanine expansion (20/25, 2 males vs 2 females), four presented with 6 additional Alanine expansion (20/26, 2 males vs 2 females), three presented with 7 additional Alanine expansion (20/27, 1 male vs 2 females), only one presented with 9 additional Alanine expansion (20/29, 1 female). None had tumors of the neural crest. One 23 years old male patient had Hirschsprung disease (PARM 20/26), a cardiac pace-maker but not a diaphragm pacing at the time of the study. All of them required ventilatory support only during sleep, while none required full-time assisted ventilation at the time of the study. None had assisted ventilation during the day, such as diaphragm pacing, at the time of the study. None had tracheostomy at the time of the study: nevertheless, one female patient had tracheostomy and had required daytime artificial ventilation until age of 8, one female patient had tracheostomy and had required daytime artificial ventilation until age of 10, one female patient had tracheostomy and had required daytime artificial ventilation until age of 15, one male patient had tracheostomy and had required daytime artificial ventilation until age of 16, one female patient had tracheostomy and had required daytime artificial ventilation until age of 17.
All patients were in fact ventilated overnight. All patients were ventilated using life support ventilator (Astral ventilator) with a pressure support mode and in approximately a third of cases with a targeted volume. All patients had a high backup rate (generally at least a minimum of 16 breaths/min). The level of expiratory pressure was usually set at 4 cmH2O and the inspiratory pressure at 18 cmH2O. Most of our cohort was ventilated using oronasal mask and none of them had oxygen entrained in their ventilator. All patients that underwent CPET had satisfactory control of overnight breathing. All patients were rigorously adherent to non-invasive ventilation use (approximately 8 hrs/day of use).
In addition, all CCHS patients were lifelong non-smokers. None had asthma, one had pulmonary arterial hypertension, two had arterial hypertension, three had cardiac pace-makers. None of these abnormalities contra-indicated CPET 26. Pulmonary function tests and room air arterial blood gases were normal (Table 1): none had elevated PETCO2 or PaCO2 at rest before exercise or low oxygen saturations (SpO2 or PaO2). The CO2 response was reduced compared with normal values published in the literature.
Ventilatory, breathing pattern and respiratory mechanics response to CPETThe physiological responses to CPET are detailed in Tables 2 and 3.
Physiological responses to cardiopulmonary exercise testing at rest and at peak in CCHS and healthy subjects.
| Rest | Peak | |||
|---|---|---|---|---|
| Variables | CCHS | Healthy | CCHS | Healthy |
| Work rate, watts | 0 (0 – 0) | 0 (0 – 0) | 90 (80 – 130)⁎⁎⁎ | 190 (155 – 240) |
| V'O2, L/min | 0.321 (0.296 – 0.352) | 0.282 (0.273 – 0.303) | 1.472 (1.160 − 1.790)⁎⁎⁎ | 2.555 (2.070 − 2.890) |
| V'O2, % pred | 15 (13 – 16) | 12 (11 – 14) | 68 (44 − 80)⁎⁎⁎ | 99 (98 − 102) |
| SpO2, % | 98 (96 – 99) | 99 (99 – 99) | 91 (86 − 95)⁎⁎⁎ | 99 (98 − 99) |
| PaO2, mmHg | 105 (94 – 115) | / | 78 (71 − 91) | / |
| PaCO2, mmHg | 36 (29 – 40) | / | 54 (48 − 60) | / |
| PETCO2, mmHg | 33 (28 – 36) | 39 (38 – 40) | 59 (50 − 65)⁎⁎⁎ | 40 (37 − 42) |
| V’E, L/min | 9.8 (9.2 – 11.9) | 9.9 (9.0 – 10.2) | 26.3 (20.6 − 40.9)⁎⁎⁎ | 106.5 (97.2 − 110.5) |
| VT, L | 0.58 (0.48 – 0.71) | 0.60 (0.58 – 0.68) | 1.21 (0.92 − 1.40)⁎⁎⁎ | 2.94 (2.73 − 3.05) |
| Bf, breaths/min | 17 (15 – 22) | 15 (15 – 17) | 27 (19 − 30)⁎⁎⁎ | 37 (32 − 40) |
| IRV, L | 1.89 (1.69 – 2.77) | 2.59 (2.31 – 2.78) | 1.36 (0.84 − 1.94)⁎⁎⁎ | 0.59 (0.40 − 0.77) |
| ∆ IRV, L | 0 (0 – 0) | 0 (0 – 0) | −0.95 (−1.06 − −0.39)⁎⁎⁎ | −1.85 (−2.16 − −1.44) |
| ERV, L | 1.40 (1.03 – 1.63) | 1.83 (1.34 – 2.08) | 1.86 (1.04 − 2.09)* | 1.44 (0.89 − 1.50) |
| ∆ ERV, L | 0 (0 – 0) | 0 (0 – 0) | 0.06 (−0.06 − 0.53)* | −0.45 (−0.60 − −0.39) |
| IC, L | 2.51 (2.13 – 3.44) | 3.20 (2.90 – 3.36) | 2.50 (2.02 − 3.29)⁎⁎⁎ | 3.62 (3.42 − 3.83) |
| ∆ IC, L | 0 (0 – 0) | 0 (0 – 0) | −0.06 (−0.53 − 0.06)⁎⁎⁎ | 0.45 (0.39 − 0.60) |
Results are presented as median (IQR 25 %−75 %). CCHS: congenital central hypoventilation syndrome; V'O2: oxygen uptake; SpO2: pulse oximetry oxygen saturation; PETCO2: end-tidal carbon dioxide partial pressure; PaO2, PaCO2: partial pressure of oxygen/carbon dioxide in arterial blood, respectively; V’E: ventilation; Bf: breathing frequency; VT: tidal volume; IRV: inspiratory reserve volume; ERV: expiratory reserve volume; IC: inspiratory capacity; ∆: the change or difference from rest; pred: predicted.
Physiological responses to cardiopulmonary exercise testing at iso-WR (40 W) and iso-V’E (15 L/min) in CCHS and healthy subjects.
| iso-V’E (15 L/min) | iso-WR (40 W) | |||
|---|---|---|---|---|
| Variables | CCHS | Healthy | CCHS | Healthy |
| Work rate, watts | 16 (14 – 30) | 26 (19 – 29) | 40 | 40 |
| V'O2, L/min | 0.613 (0.491 − 0.670) | 0.499 (0.443 − 0.545) | 0.841 (0.750 – 0.912)* | 0.660 (0.603 – 0.785) |
| V'O2, % pred | 28 (20 − 33) | 21 (20 − 23) | 35 (26 – 42)* | 27 (25 – 31) |
| SpO2,% | 96 (92 − 98)* | 99 (99 − 99) | 96 (93 – 98)* | 99 (99 – 99) |
| PETCO2, mmHg | 38 (34 − 46) | 41 (40 − 42) | 45 (38 – 51) | 41 (40 – 42) |
| V’E, L/min | 15.0 | 15.0 | 17.6 (15.4 – 22.0) | 17.4 (17.1 – 19.4) |
| VT, L | 0.72 (0.68 − 0.80)* | 0.93 (0.76 − 0.97) | 0.92 (0.73 – 1.01)* | 1.04 (0.94 – 1.12) |
| Bf, breaths/min | 21 (19 − 22)* | 16 (16 − 20) | 21 (18 – 23)* | 17 (15 – 19) |
| IRV, L | 1.68 (1.37 − 2.73) | 2.54 (1.96 − 2.75) | 1.74 (1.45 – 2.52) | 2.40 (1.93 – 2.71) |
| ∆ IRV, L | −0.14 (−0.19 − −0.02)⁎⁎ | −0.06 (−0.17 − 0.01) | −0.28 (−0.47 − −0.24)⁎⁎ | −0.15 (−0.24 – −0.01) |
| ERV, L | 1.45 (1.06 − 1.60) | 1.69 (1.03 − 1.85) | 1.52 (1.29 – 2.08) | 1.64 (0.96 – 1.76) |
| ∆ ERV, L | 0.00 (−0.04 − 0.04)⁎⁎ | −0.14 (−0.31 − −0.10) | −0.02 (−0.07 – 0.20)⁎⁎ | −0.24 (−0.32 – −0.19) |
| IC, L | 2.51 (2.15 − 3.45)⁎⁎ | 3.36 (3.18 − 3.48) | 2.62 (2.18 – 3.52)⁎⁎ | 3.43 (3.34 – 3.62) |
| ∆ IC, L | 0.00 (−0.04 – 0.04)⁎⁎ | 0.14 (0.10 − 0.31) | 0.02 (−0.20 – 0.07)⁎⁎ | 0.24 (0.19 – 0.32) |
Results are presented as median (IQR 25 %−75 %). CCHS: congenital central hypoventilation syndrome; iso-V’E: common standardized ventilation (V’E) of 15 L/min; iso-WR: common standardized exercise work-rate (WR) of 40 W; V'O2: oxygen uptake; SpO2: pulse oximetry oxygen saturation; PETCO2: end-tidal carbon dioxide partial pressure; V’E: ventilation; Bf: breathing frequency; VT: tidal volume; IRV: inspiratory reserve volume; ERV: expiratory reserve volume; IC: inspiratory capacity; ∆: the change or difference from rest; pred: predicted.
In the CCHS patients, the criteria for CPET interruption 26 were SpO2 falling below 90 % in 5 patients, PETCO2 > 50 mmHg in 8 patients, visual blur, headache or eyes tingling in 4 patients, leg fatigue in 2 patients, and dyspnoea in 2 patients. Healthy subjects had normal exercise capacity and consistently stopped exercising due to intolerable exertional leg discomfort.
Peak V'O2 and WR were significantly lower in CCHS patients than controls. V’E and VT increased in both CCHS and controls up to 40 W; thereafter V’E and VT decreased consistently in CCHS patients but continued to increase in controls. PETCO2 was comparable between CCHS and healthy subjects up to 40 W; thereafter it increased significantly in CCHS patients until peak exercise and remained stable in controls.
Inspiratory capacity (IC) increased throughout exercise in controls (Fig. 2A), but decreased by 0.59 L in 5 CCHS patients, increased by 0.23 L in 2 CCHS patients, and stayed the same as at rest in 6 CCHS patients. Expiratory reserve volume (ERV) decreased consistently throughout exercise in controls (Fig. 2A) (tidal expiratory recruitment). In contrast, in CCHS patients, ERV increased between rest and 40 W and then stabilised until peak exercise (Fig. 2B) (defective or absent expiratory recruitment). IRV decreased consistently in both CCHS and controls from rest to peak (Fig. 2A and B) (tidal inspiratory recruitment) and was more marked in CCHS patients than controls (Fig. 2). Of note, 2/13 CCHS patients did not show oxygen desaturation or hypercapnia during exercise: nonetheless, the behavior of their ERV was the same as the remaining CCHS patients who showed significant oxygen desaturation or hypercapnia on exertion. Last but not least, in CCHS patients there were no differences between the sexes in terms of ventilatory, VT and IRV-ERV recruitment profile response to exercise.
Inspiratory reserve flow at peak exercise was not statistically different between CCHS and healthy subjects (1.8 vs 2.0 L/s, p = 0.7). Finally, there was no evidence of expiratory flow limitation at rest or during exercise in CCHS patients or healthy controls.
DiscussionDuring exercise under standard CPET conditions, CCHS patients showed subnormal maximal oxygen uptake and reduced exercise tolerance. In most patients (11/13) the occurrence of hypercapnia during CPET indicated ventilatory limitation to exercise.30 In comparison to normal subjects who increased ventilation in response to exercise through both inspiratory and expiratory reserves, CCHS patients showed an abnormal ventilatory response without any indication of expiratory muscle recruitment.
In healthy individuals, alveolar ventilation (and thus V'E) rises linearly as a function of V'CO2 so that alveolar carbon dioxide and oxygen partial pressures (PACO2 and PAO2) and their arterial counterparts (PaCO2 and PaO2) are maintained near resting values throughout exercise.31 This involves: (i) mechanical and chemical feedback to medullary respiratory pattern generators adjusting respiratory muscle activity, and (ii) feed-forward mechanisms from higher neural centers driving the medullary generators and increasing locomotor muscle activity.32 In animals, two medullary generators drive inspiration (the preBötC) and expiration (the pFRG).2,33 Animal data show that, during exercise, the RTN, adjacent to the pFRG, is involved in adapting V’E in response to increased V'CO2 by augmenting expiratory activity,7,8 possibly via PHOX2B-positive neuronal projections to the pFRG.34 In healthy humans, V’E initially increases during exercise through increases in VT that normally expands to 50–60 % of vital capacity by encroaching on the IRV and ERV. The latter involves the early activation of expiratory muscles and results in reductions in ERV that vary with the type and intensity of exercise.16,25,31,35,36 This was observed in our control cohort. The recruitment of expiratory muscles directly increases V’E and lengthens respiratory muscles, optimizing their action and placing them at mechanical advantage, as well as storing chest wall elastic recoil energy to be released at the beginning of inspiration.37 This IRV-ERV recruitment pattern, clearly visible in our control group, was lacking in our CCHS patients in whom ERV increased (rather than decreased) from rest to 40 W. From 40 W to peak exercise ERV continued to decrease in controls but remained stable in CCHS patients. In contrast, inspiratory recruitment was higher in CCHS patients than controls. These differences were best seen when comparing CCHS and healthy controls at the same ventilatory stimulus, i.e. at iso-V’E, (Table 3 and Fig. 2). The same profile was observed with regards to V'E and VT response to exercise: V'E and VT increased in CCHS patients until 40 W, this being in line with previous published literature,14 and then decreased, which was not observed in the healthy participants (p<0.001).
Several mechanisms can impede exercise-induced expiratory reserve recruitment. Expiratory flow limitation (forced or exercise-induced expiratory flows equal to or lower than tidal expiratory flow)29 results from the bronchi closing prematurely in response to the expiratory driving pressure and is typically seen in chronic obstructive pulmonary disease (COPD)38 and in obesity.38 This was not observed in our study and has not been described in the literature for CCHS patients. In addition, the shape of the maximal flow-volume loop at rest was completely normal in CCHS patients, so that no expiratory flow limitation was observed at rest. During exercise VT increased only by encroaching on the IRV and not on the ERV, so that a flow reserve was still available on the expiratory portion of the maximal flow-volume loop, indicating that no expiratory flow limitation was noted in CCHS. Expiratory muscle weakness or fatigue can also limit expiratory reserve recruitment, as seen in patients with paraplegia.38 This mechanism does not seem likely in CCHS patients, who have normal lung function at rest. Inspiratory muscle weakness or fatigue reducing IC can give a false impression of impeded recruitment of expiratory reserve when, during CPET, ERV is calculated as resting FVC minus IC. Inspiratory muscle fatigue during exercise in our patients is made unlikely by the absence of any alteration of inspiratory reserve flow at peak exercise.29
Central neurological mechanisms can also be invoked. Although medullary sites have been identified in humans that could be homologous to the preBötC39 and RTN,15 the organisation and functioning of the respiratory network in humans is not necessarily similar to that described in rodents nor are the consequences of PHOX2B mutations necessarily the same in humans as in rodents. However, blunted CO2 chemosensitivity and a blunted ventilatory response to exercise characterized by defective expiratory recruitment that we have shown would be fully compatible with RTN dysfunction. In our study, CCHS patients showed a residual CO2 ventilatory response and responded to exercise with a progressive increase in VT encroaching on IRV, although all required ventilatory support during sleep. This indicates the persistence of wakefulness-related automatic rhythmogenesis. This may appear contradictory to the hypothesis of RTN dysfunction, with interactions between the RTN and the preBötC complex being necessary for the latter to produce inspiration.1 However, CCHS patients are known to activate respiratory-related cortical networks during wakefulness,40,41 a mechanism that is believed to maintain breathing through cortico-subcortical cooperation. In the hypothesis of RTN deficiency, the function of the cortical networks would be to allow the RTN-preBötC interaction to function despite the RTN defect, a possibility that could depend on the existence of some residual PHOX2B-positive cells, as has been observed in animals.6
LimitationsOur study has some limitations. The relatively small sample size means that we must be circumspect about generalising our findings to the wider CCHS population. However, we have performed a phenomenological study exploring a unique series of 13 adult patients (which is not inconsiderable with this disease) which represented a “genotypically and phenotypically homogeneous set of patients”.
Respiratory muscle function was not assessed in our study to formally rule out respiratory muscle weakness or fatigue, but our choice was justified by the exploratory nature of the study and our concern to keep it as close to observational as possible. Expiratory muscle weakness or fatigue can limit expiratory reserve recruitment. This mechanism does not seem likely in CCHS patients, who have normal lung function at rest. In addition, maximal expiratory pressure (MEP) was measured only in 3 CCHS patients (data not shown) and found normal. Further studies should include MEP measures. Inspiratory muscle weakness or fatigue reducing IC can give a false impression of impeded recruitment of expiratory reserve when, during CPET, ERV is calculated as resting FVC minus IC. Inspiratory muscle fatigue during exercise in our CCHS patients is made unlikely by the absence of any alteration of inspiratory reserve flow at peak exercise. In addition, maximal inspiratory pressure (MIP) and Sniff Nasal Inspiratory Pressure (SNIP) were measured in 7 CCHS patients (data not shown) and found normal. Further studies should include MIP and SNIP measures.
Blood gases at rest and the ventilatory response to CO2 were not available for healthy controls as they were selected from a healthy-control database used in previous publications17,18 in which blood gases and the ventilatory response to CO2 were not performed. Further studies containing this information are needed.
Further studies with a larger sample size and engaging more detailed esophageal, gastric and transdiaphragmatic pressure measurements during exercise will be required to definitively elucidate the physiological mechanisms of the altered ventilatory mechanics seen in CCHS patients.
ConclusionsIn conclusion, while our study is phenomenological and does not provide formal proof of the hypothesised mechanism, we have shown defective exercise-induced expiratory recruitment in CCHS patients. The consistency of the observations made across a genotypically and phenotypically homogeneous set of patients, and their coherence with animal data, are of heuristic interest. For example, the possibility of improving breathing control pharmacologically was raised by observations of restored CO2 responses under contraceptive treatment by desogestrel.42 This has prompted various projects investigating pharmacological interventions to improve respiratory rhythmogenesis.43–45 Our data, by refining the phenotypic description of breathing control in CCHS patients, suggest the use of the ERV response to exercise as a possible novel outcome for such investigations.
Author's contributions to the studyPL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. QF: Data curation, Formal analysis, Investigation, Writing - review & editing. MB: Data curation, Formal analysis, Writing - review & editing. MP: Investigation, Project administration, Writing - review & editing. BD: Investigation, Writing - review & editing. CL: Project administration, Writing - review & editing. CMP: Project administration, Writing - review & editing. FC: Methodology, Writing - review & editing. LB: Methodology, Writing - review & editing. CS: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. TS: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
We declare that Artificial Intelligence program has not been used in writing this article.
PL reports personal fees and support for attending meetings and/or travel from Chiesi, AstraZeneca and GSK, outside of this work. CMP reports personal fees from Chiesi, ADEP, Vivisol, Astra Zeneca, Menarini, SOS Oxygène and Resmed, outside of this work. MP reports grants from Fisher & Paykel, Resmed and Asten Santé, personal fees from Philips Respironics, Resmed, Asten Santé, GSK, Air Liquide Medical, SOS Oxygène, Jazz Pharmaceutical, Antadir, Loewenstein and Chiesi, support for attending meetings and/or travel from Asten Santé, stock from Kernel Biomedical and receipt of equipment, materials, drugs, medical writing, gifts or other services from Philips Respironics, Resmed and Fisher & Paykel, outside of this work. TS reports personal fees from ADEP assistance, Astra Zeneca, Chiesi, KPL consulting, Lungpacer Inc., OSO-AI, Vitalaire, TEVA, patents (WO2008006963A3, WO2012004534A1, WO2013164462A1) and stock from AUSTRAL Dx and HEPHAI, outside of this work. CS, CL, QF, MB, LB, FC, BD report no conflicts of interests.







![Summary of the study hypothesis. Panel A. Anatomical organization of the brainstem respiratory network in humans (left and top), inferred from analogies with rodent data and human data (see Schwarzacher, S. W., U. Rub and T. Deller 2011, reference 39 in the manuscript for the putative localization of the human pre-Bötzinger complex (preBötC); see Levy, J., F. Droz-Bartholet, M. Achour, P. Facchinetti, B. Parratte and F. Giuliano 2021, reference 15 in the manuscript for the putative location of the retrotrapezoid nucleus [RTN]). The image shows the localisation of the hypoglossal motor nucleus (XII), the nucleus ambiguus, the facial motor nucleus (VII), the inferior olive (IO), the lateral reticular nucleus (LRN), the parafacial respiratory group (pFRG) which contains the ventral parafacial nucleus (pFV, originally and still most often referred to as the RTN) and the lateral parafacial nucleus (pFL), and the ventral respiratory column (VRC) which contains the preBötC. The Fig. follows the hypothesis that the preBötC mostly drives inspiratory muscles (diaphragm and external intercostal muscles, shown in red) and that the pFRG mostly drives expiratory muscles (internal intercostal and abdominal muscles, shown in blue). Panel B. Conceptual representation of the organisation of respiratory central pattern generators, with inspiratory-related circuits shown in red and expiratory-related circuits shown in blue. The rostral ventral respiratory group (rVRG) contains inspiratory neurons and premotor neurons. The caudal ventral respiratory group (cVRG) contains expiratory premotor neurons. This organisation mainly involves three essential components: 1) an (inexorable) inspiratory oscillator in preBötC that drives inspiration by exciting inspiratory premotor neuronal populations, e.g., rVRG and parahypoglossal region (XII), and inhibits pFL; 2) a (conditional) expiratory oscillator in pFL that gates and drives expiration by exciting expiratory premotor neuronal populations, such as cVRG and XII, and assures alteration of phases by exciting neurons that inhibit preBötC, e.g., inhibitory neurons in either the preBötC or BötC; and 3) a source of tonic drive in pFV (RTN) which derives from dorsal progenitor cells that express paired-like homeobox 2B (PHOX2B) that are able to detect signals related to CO2 and/or pH levels (possibly with the involvement of glia) and integrates other sensory afferents affecting respiratory drive, via excitatory connections to preBötC, BötC, and respiratory premotor neurons, such as rVRG, cVRG, and XII. Respiratory muscles are activated at a certain frequency (FB) and intensity to generate tidal volume (VT). The excitation of rVRG activates inspiratory muscles that produce the inspired component of tidal volume (VTi), at rest and its exercise-related increases. The excitation of cVRG activates expiratory muscles that produce the expired component of tidal volume (VTe), particularly during exercise. Panel C. Tidal volume (VT) dynamics at rest and during exercise in healthy humans (“controls”, top) and patients with congenital central hypoventilation syndrome (“CCHS”, bottom). At rest, inspiration is active and expiration is passive, breathing therefore occurring above functional residual capacity (FRC) defined as the respiratory system relaxation volume (dotted horizontal line). An inspiratory reserve volume (IRV) separates the end of tidal inspiration from full inspiration, and an expiratory reserve volume (ERV) separates the end of tidal expiration (i.e. FRC) from full expiration. The human ventilatory response to exercise is primarily achieved through an increase in tidal volume (VT) that encroaches on both IRV and ERV through the recruitment of inspiratory and expiratory muscles, as depicted on the top trace. Assuming that the human organisation of respiratory rhythmogenesis is similar to that described in rodents, we reasoned that PHOX2B-related RTN/pFRG dysfunction in CCHS patients would result in defective exercise-induced expiratory recruitment. The figure is original and based on a drawing commissioned to and contributed by artist Pierre Bourcier (Paris, France). Summary of the study hypothesis. Panel A. Anatomical organization of the brainstem respiratory network in humans (left and top), inferred from analogies with rodent data and human data (see Schwarzacher, S. W., U. Rub and T. Deller 2011, reference 39 in the manuscript for the putative localization of the human pre-Bötzinger complex (preBötC); see Levy, J., F. Droz-Bartholet, M. Achour, P. Facchinetti, B. Parratte and F. Giuliano 2021, reference 15 in the manuscript for the putative location of the retrotrapezoid nucleus [RTN]). The image shows the localisation of the hypoglossal motor nucleus (XII), the nucleus ambiguus, the facial motor nucleus (VII), the inferior olive (IO), the lateral reticular nucleus (LRN), the parafacial respiratory group (pFRG) which contains the ventral parafacial nucleus (pFV, originally and still most often referred to as the RTN) and the lateral parafacial nucleus (pFL), and the ventral respiratory column (VRC) which contains the preBötC. The Fig. follows the hypothesis that the preBötC mostly drives inspiratory muscles (diaphragm and external intercostal muscles, shown in red) and that the pFRG mostly drives expiratory muscles (internal intercostal and abdominal muscles, shown in blue). Panel B. Conceptual representation of the organisation of respiratory central pattern generators, with inspiratory-related circuits shown in red and expiratory-related circuits shown in blue. The rostral ventral respiratory group (rVRG) contains inspiratory neurons and premotor neurons. The caudal ventral respiratory group (cVRG) contains expiratory premotor neurons. This organisation mainly involves three essential components: 1) an (inexorable) inspiratory oscillator in preBötC that drives inspiration by exciting inspiratory premotor neuronal populations, e.g., rVRG and parahypoglossal region (XII), and inhibits pFL; 2) a (conditional) expiratory oscillator in pFL that gates and drives expiration by exciting expiratory premotor neuronal populations, such as cVRG and XII, and assures alteration of phases by exciting neurons that inhibit preBötC, e.g., inhibitory neurons in either the preBötC or BötC; and 3) a source of tonic drive in pFV (RTN) which derives from dorsal progenitor cells that express paired-like homeobox 2B (PHOX2B) that are able to detect signals related to CO2 and/or pH levels (possibly with the involvement of glia) and integrates other sensory afferents affecting respiratory drive, via excitatory connections to preBötC, BötC, and respiratory premotor neurons, such as rVRG, cVRG, and XII. Respiratory muscles are activated at a certain frequency (FB) and intensity to generate tidal volume (VT). The excitation of rVRG activates inspiratory muscles that produce the inspired component of tidal volume (VTi), at rest and its exercise-related increases. The excitation of cVRG activates expiratory muscles that produce the expired component of tidal volume (VTe), particularly during exercise. Panel C. Tidal volume (VT) dynamics at rest and during exercise in healthy humans (“controls”, top) and patients with congenital central hypoventilation syndrome (“CCHS”, bottom). At rest, inspiration is active and expiration is passive, breathing therefore occurring above functional residual capacity (FRC) defined as the respiratory system relaxation volume (dotted horizontal line). An inspiratory reserve volume (IRV) separates the end of tidal inspiration from full inspiration, and an expiratory reserve volume (ERV) separates the end of tidal expiration (i.e. FRC) from full expiration. The human ventilatory response to exercise is primarily achieved through an increase in tidal volume (VT) that encroaches on both IRV and ERV through the recruitment of inspiratory and expiratory muscles, as depicted on the top trace. Assuming that the human organisation of respiratory rhythmogenesis is similar to that described in rodents, we reasoned that PHOX2B-related RTN/pFRG dysfunction in CCHS patients would result in defective exercise-induced expiratory recruitment. The figure is original and based on a drawing commissioned to and contributed by artist Pierre Bourcier (Paris, France).](https://static.elsevier.es/multimedia/25310437/unassign/S2531043724000096/v1_202402260343/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)