Administration of supplemental oxygen is a life-saving treatment in critically ill patients. Still, optimal dosing remains unclear during sepsis. The aim of this post-hoc analysis was to assess the association between hyperoxemia and 90-day mortality in a large cohort of septic patients.
MethodsThis is a post-hoc analysis of the Albumin Italian Outcome Sepsis (ALBIOS) randomized controlled trial (RCT). Patients with sepsis who survived the first 48 h since randomization were included and stratified into two groups according to their average PaO2 levels during the first 48 h (PaO2 0–48 h). The cut-off value was established at 100 mmHg (average PaO2 0–48 h >100 mmHg: hyperoxemia group; PaO2 0–48h≤100: normoxemia group). The primary outcome was 90-day mortality.
Results1632 patients were included in this analysis (661 patients in the hyperoxemia group, 971 patients in the normoxemia group). Concerning the primary outcome, 344 (35.4%) patients in the hyperoxemia group vs. 236 (35.7%) in the normoxemia group had died within 90 days from randomization (p = 0.909). No association was found after adjusting for confounders (HR 0.87; CI [95%] 0.736–1.028, p = 0.102) or after excluding patients with hypoxemia at enrollment, patients with lung infection or including post-surgical patients only. Conversely, we found an association between lower risk of 90-day mortality and hyperoxemia in the subgroup including patients who had the lung as primary site of infection (HR 0.72; CI [95%] 0.565–0.918). Mortality at 28 days, ICU mortality, incidence of acute kidney injury, use of renal replacement therapy, days to suspension of vasopressor or inotropic agents, and resolution of primary and secondary infections did not differ significantly. Duration of mechanical ventilation and length of stay in ICU were significantly longer in patients with hyperoxemia.
ConclusionsIn a post-hoc analysis of a RCT enrolling septic patients, hyperoxemia as average PaO2>100 mmHg during the first 48 h was not associated with patients’ survival.
Administration of supplemental oxygen is a life-saving treatment in critically ill patients with hypoxemia,1 but optimal dosing remains unclear in various patient populations. Critically ill patients treated with excessive doses of supplemental oxygen may develop hyperoxemia, which may have detrimental effects on organs and tissues. Hyperoxemia reduces heart rate, cardiac output and coronary perfusion, and increases systemic vascular resistance.2 Moreover, hyperoxemia decreases cerebral blood flow and causes pulmonary toxicity through different mechanisms (altered surfactant protein composition, reduced mucociliary clearance, inflammation of lung parenchyma, resorption atelectasis in lung regions with low ventilation/perfusion ratios, reduced lung compliance and increased risk of infections).1,3,4 On the other hand, higher levels of oxygen may boost a neutrophilic response to extracellular pathogens through oxidative killing, suggesting that hyperoxemia could be associated with a lower risk of surgical site infections and consequently with a survival benefit in patients undergoing major surgery. 5,6
The effect of O2 supplement has been studied in intensive care unit population 7 as well as in more specific categories of patients such as invasively mechanically ventilated patients8,9 or patients undergoing ECMO,10 but there is still conflicting evidence regarding the use of conservative or liberal oxygen administration in septic patients.5,11–13 Therefore, the aim of this post-hoc analysis was to evaluate the association between hyperoxemia, mortality and other clinical outcomes in septic patients. The hypothesis tested was that hyperoxemia may be associated with lower 90-day mortality.
MethodsStudy cohortThis study is a post-hoc analysis of the trial Albumin Italian Outcome Sepsis (ALBIOS), prospectively registered on 30 June 2008 - NCT00707122.14 The ALBIOS trial was a multicenter, open-label, randomized, controlled trial (RCT) that enrolled 1810 patients with severe sepsis or septic shock. Adult patients who met the clinical criteria for severe sepsis or septic shock within the previous 24 h were screened for eligibility criteria and enrolled in the ALBIOS trial. Patients were randomly assigned to receive either 20% albumin and crystalloid solution or crystalloid solution alone for fluid replacement from randomization until day 28 or ICU discharge. All the other treatments were administered according to the standard guidelines of the treatment of septic patients. The study found that albumin replacement in addition to crystalloids, as compared to crystalloids alone, did not improve the survival at 28 or 90 days14,15
For this post-hoc analysis, patients who had died within 48 h after randomization were excluded as well as patients whose data was incomplete regarding partial pressure of arterial oxygen (PaO2) values at all timepoints. Recorded values of PaO2 expressed in millimeters of mercury (mmHg) were collected at different time points during the first 48 h. Patients were stratified into two groups according to their weighted average PaO2 levels, the cut-off value was established at 100 mmHg as in previous literature5,16: patients with average PaO2 in the first 48 h (PaO2 0–48 h)>100 mmHg were assigned to the hyperoxemia group. Conversely patients with PaO2 0–48h≤100 mmHg were included in the normoxemia group.
OutcomesThe primary outcome was 90-day mortality. Secondary outcomes were 28-day mortality, ICU mortality, duration of mechanical ventilation, length of stay in ICU, incidence of renal-replacement therapy and Acute Kidney Injury (AKI) defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria,17 days to suspension of vasopressor or inotropic agents, length of stay in ICU, and the percentage of resolution of primary and secondary infections.
Statistical analysisPatients were dichotomized based on average PaO2≤100 mmHg or >100 mmHg per hour for the first 48 h after randomization. PaO2 was available at baseline, 6, 24 and 48 h, The area under the curve was calculated and divided by 48 to have the average PaO2 value per hour.
Continuous variables were expressed as means +/- Standard Deviation (SD) when normally distributed, as median and interquartile range [IQR] when non-normally distributed. Categorical data were reported as frequencies and proportions. The Pearson Chi2 test, one-way ANOVA test, or Kruskal-Wallis test were used to examine differences between the two groups.
The associations between the groups and mortality at 90 days were presented by Kaplan-Meier. The Log-Rank test was applied to compare the survival distribution of the groups. Cox regression analysis was performed to estimate the hazard-ratio associated with the risk of mortality, adjusted for the variables selected considering baseline unbalances that were statistically and clinically relevant and independent from each other: type of admission, mechanical ventilation, shock (according to Sepsis-2 definition18), chronic renal failure and Simplified Acute Physiology Score II (SAPS II).
Five subgroup analyses were performed. In the first analysis, patients with hypoxemia (PaO2≤60 mmHg) at baseline were excluded, as done in previous literature in order to avoid the possible confounding effect of extremely low values of PaO25. The second subgroup analysis only focused on septic patients admitted to ICU after emergency and elective surgery, to evaluate the possible benefit of hyperoxia in this specific cohort of patients. Two other sub-group analyses were performed: one excluding patients with lung as primary site of infection and another one only including patients with lung as primary site of infection, to observe how the infection site would influence survival. Lastly, we performed a subgroup analysis including patients who underwent mechanical ventilation.
ResultsBaseline characteristicsThe ALBIOS trial included 1810 patients diagnosed with severe sepsis or septic shock. After the exclusion of 174 patients who died in the first 48 h, and 4 patients due to incomplete PaO2 values, 1632 patients were included in this analysis (Supplementary Fig. 1). Information on the characteristics of excluded patients can be found in Supplementary Table 1. Baseline characteristics of the cohort included are shown in Table 1.
Baseline characteristics of included patients.
| Overall N = 1632 | PaO2 0–48 h ≤ 100 mmHgN = 661 (40.7%) | PaO2 0–48h>100 mmHg for 48 h N = 971 (59.3%) | P* | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Albumin | N (%) | 805 (49.3) | 330 (49.9) | 475 (48.9) | 0.689 | |
| Sex | Female | N (%) | 628 (38.5) | 250 (37.8) | 378 (38.9) | 0.651 | |
| Age | year | Mean (SD) | 66 (14) | 66 (14) | 66 (15) | 0.997 | |
| BMI | kg/m2 | Mean (SD) | 27 (6) | 26.9 (5) | 26.4 (6) | 0.107 | |
| Reason for admission | Medical | N (%) | 914 (56.0) | 388 (58.6) | 526 (54.2) | 0.071 | |
| Elective | N (%) | 116 (7.1) | 55 (8.3) | 61 (6.3) | 0.115 | ||
| Emergency | N (%) | 602 (36.9) | 218 (32.9) | 384 (39.5) | 0.007 | ||
| MAP | mmHg | Median [IQR] | 73.3 [63.3–83.3] | 72.3 [63–83.3] | 73.3 [63.3–83.3] | 0.298 | |
| Heart Rate | bpm | Median [IQR]) | 105 [91–120] | 105 [90–120] | 105 [92–120] | 0.782 | |
| Urine output | ml/h | Median [IQR] | 60 [30–100] | 60 [30–100] | 50 [30–100] | 0.120 | |
| SAPS II score | Median [IQR] | 46 [36–57] | 45 [35–56] | 47 [36–58] | 0.041 | ||
| hemoglobin | g/dl | Median [IQR] | 10.8 [9.6–12.2] | 11 [9.7–12.5] | 10.7 [9.5–12.1] | 0.0006 | |
| Arterial Oxygen Saturation | % | Median [IQR] | 97 [95–99] | 96 [94–98] | 98 [96–99] | <0.0001 | |
| Central Venous Oxygen Saturation | % | Median [IQR] | 73 [67–79] | 71 [65–78] | 74 [69–80] | <0.0001 | |
| Central venous pressure | mmHg | Median [IQR] | 10 [7–13] | 10 [6–13] | 10 [7–13] | 0.642 | |
| Mechanical ventilation | N (%) | 1290 (79) | 483 (73.1) | 807 (83.1) | <0.0001 | ||
| FiO2 | % | Median [IQR] | 50 [50–70] | 55 [45–70] | 50 [50–70] | 0.761 | |
| Congestive/ischemic heart disease | N (%) | 283 (17.3) | 114 (17.3) | 169 (17.4) | 0.934 | ||
| Liver disease | N (%) | 23 (1.4) | 9 (1.4) | 14 (1.4) | 0.892 | ||
| Immunodeficiency | N (%) | 204 (12.5) | 82 (12.4) | 122 (12.6) | 0.924 | ||
| Chronic renal failure | N (%) | 63 (3.9) | 18 (2.7) | 45 (4.6) | 0.049 | ||
| COPD | N (%) | 194 (11.9) | 90 (13.6) | 104 (10.7) | 0.075 | ||
| Active hematologic malignancy | N (%) | 63 (3.9) | 28 (4.2) | 35 (3.6) | 0.515 | ||
| Active neoplasm | N (%) | 58 (3.5) | 20 (3.0) | 38 (3.9) | 0.341 | ||
| AIDS | N (%) | 15 (0.9) | 5 (0.8) | 10 (1.03) | 0.569 | ||
| Primary site of infection | Lung | N (%) | 663 (40.6) | 296 (44.8) | 367 (37.8) | 0.004 | |
| Abdomen | N (%) | 645 (39.5) | 239 (36.1) | 406 (41.8) | 0.021 | ||
| Urinary tract | N (%) | 222 (13.6) | 75 (11.4) | 147 (15.1) | 0.028 | ||
| Other site | N (%) | 296 (18.2) | 120 (18.2) | 176 (18.1) | 0.988 | ||
| Baseline lactate | mmol/L | Median [IQR] | 2.3 [1.5–3.9] | 2.2 [1.3–4] | 2.4 [1.5–3.9] | 0.353 | |
| Baseline albumin | g/L | Median [IQR] | 24 [20–29] | 25 [21–29] | 24 [20–28] | 0.024 | |
| White blood cells | baseline | Median [IQR] | 12 [5.7–18.7] | 11.6 [5.5–17.8] | 12.2 [6.1–19.2] | 0.072 | |
| day 1 | Median [IQR] | 12.3 [7.7–18.6] | 11.9 [7.2–18.4] | 12.6 [7.9–18.7] | 0.180 | ||
| day 2 | Median [IQR] | 12.3 [8.4–18.2] | 12.2 [8.3–18.6] | 12.3 [8.5–18] | 0.865 | ||
| day 7 | Median [IQR] | 12.7[9–18] | 13.4 [9–18.3] | 12.4 [9.1–17.8] | 0.241 | ||
| SOFA score | baseline | Median [IQR] | 8[5–10] | 8 [6–10] | 8 [5–10] | 0.605 | |
| Organ dysfunction | 1 organ | N (%) | 387(23.2) | 166 (25.1) | 212 (21.8) | 0.433 | |
| 2 organ | N (%) | 622(38.1) | 249 (37.7) | 373 (38.4) | |||
| 3 organ | N (%) | 428(26.2) | 164 (24.8) | 264 (27.2) | |||
| 4 organ | N (%) | 204(12.5) | 82 (12.4) | 122 (12.5) | |||
| 5 organ | N (%) | 0(0) | 0 (0) | 0 (0) | |||
| Septic shock | N (%) | 992(60.8) | 375 (56.7) | 617 (63.5) | 0.005 | ||
P value for Mann-Whitney or Chi2 test, as appropriate. AIDS - Acquired Immune Deficiency Syndrome, BMI – Body Mass Index, MAP – Mean Arterial Pressure, COPD – Chronic Obstructive Pulmonary Disease, FiO2 – Fraction of Inspired O2, SOFA score - Sequential Organ Failure Assessment score.
Patients were divided into two groups according to PaO2 values during the first 48 h since randomization. Six hundred and sixty-one patients were assigned to the normoxemia group with PaO2 0–48h≤100 mmHg, while 971 patients were allocated to the hyperoxemia group with PaO2 0–48h>100 mmHg. No significant differences were observed in the distribution of patients in the two study groups in relation to the study treatment (20% albumin and crystalloid solution or crystalloid solution alone for fluid replacement). The proportion of patients mechanically ventilated was 10% greater in the hyperoxemia group. Abdominal and urinary tract infections were more often the primary site of infection in patients with hyperoxemia. Conversely, lung was more often the primary site of infection in patients with normoxemia. The proportion of patients with septic shock was greater in the hyperoxemia group, see Table 1.
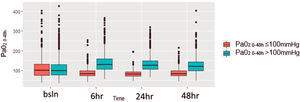
At baseline (first time point, at randomization) PaO2 values were similar between the two groups and differed significantly at 6 and 24 h from randomization (Fig. 1).
Box-plot by patient group with distribution of PaO2 values for the first 48 h.
The figure assesses the distribution of PaO2 for different timepoints during the first 48 h for patients divided in two groups according to PaO2 levels: in red patients with normoxiemia (PaO2 0–48≤100 mmHg) and in blue patients with hyperoxiemia (PaO2 0–48>100 mmHg). The horizontal line in the boxes indicates the median, the top and bottom of the box the interquartile range, and I bars the 5th and 95th percentile range.
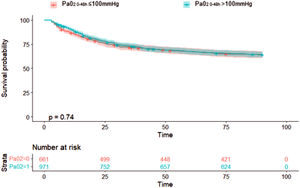
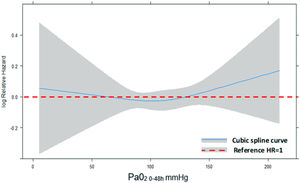
Concerning the primary outcome, 344 (35.4%) patients in the hyperoxemia group vs. 236 (35.7%) in the normoxemia group died (p = 0.909, Table 2) within 90 days from randomization. Fig. 2 shows the Kaplan-Meier curves for survival up to day 90 according to study treatment that do not differ significantly (p = 0.81). The restricted cubic splines in Fig. 3 show the trend of the hazard ratio of 90-day mortality by arterial oxygen levels. After adjusting for type of admission, mechanical ventilation, SAPS II, presence of shock and chronic renal failure, hyperoxemia was not significantly associated with 90-day mortality (HR 0.87 (CI [95%] 0.736–1.028, p = 0.102)) (Table 3). Using log-transformed PaO2 as continuous variable in the model did not change the results (Supplementary Table 2).
Primary and secondary outcomes according to study groups.
| Overall N = 1632 | PaO2 0–48h≤100 mmHgN = 661 (40.5%) | PaO2 0–48h>100 mmHg for 48 hN = 971 (59.5%) | P* | ||
|---|---|---|---|---|---|
| Death at 90 days | N (%) | 580 (35.5) | 236 (35.7) | 344 (35.4) | 0.909 |
| Death at 28 days | N (%) | 399 (24.4) | 169 (25.6) | 230 (23.7) | 0.386 |
| Renal-replacement therapy | N (%) | 366 (22.4) | 143 (21.6) | 223 (22.9) | 0.526 |
| Acute kidney injury | N (%) | 232 (14.5) | 95 (14.3) | 137 (14.2) | 0.606 |
| Duration of mechanical ventilation in days | Median [IQR] | 7 [2–25] | 6 [2–15] | 7 [3–14] | 0.036 |
| Days to suspension of vasopressor or inotropic agents | Median [IQR] | 2 [1–4] | 2 [1–5] | 2 [1–4] | 0.928 |
| Length of stay – days in ICU | Median [IQR] | 10 [5–20] | 9 [5–20] | 10 [6–19] | 0.007 |
| ICU mortality | N (%) | 433 (26.5) | 186 (28.1) | 247 (25.4) | 0.225 |
| Resolution primary infection | N (%) | 906 (55.5) | 355 (53.7) | 551 (56.7) | 0.225 |
| Secondary infection | N (%) | 404 (24.8) | 158 (23.9) | 246 (25.3) | 0.510 |
Survival analyses for 90-day mortality in PaO2 groups (above or below 100 mmHg).
SAPS II – Simplified Acute Physiology Score II. HR – Hazard ratio, CI – Confidence interval. * P value for Cox proportional hazards regression analysis.
Mortality at 28 days was similar in the two groups. AKI, proportion of patients who underwent renal-replacement therapy, timing of suspension of vasopressor or inotropic agents, resolution of primary infection and ICU mortality were not significantly different between the study groups. Patients with normoxemia underwent mechanical ventilation for a shorter period of time and had a shorter length of stay in ICU, compared to the hyperoxemia group (Table 2).
Subgroup analysesAfter excluding 112 patients with a PaO2≤60 mmHg at baseline, the adjusted association between log-transformed continuous PaO2 and 90-day mortality was not significant (Supplementary Table 3).
In the cohort of septic patients admitted after emergency or elective surgery (n = 718), hyperoxemia was not associated with lower or higher 90-day mortality (Supplementary Table 4A). Secondary outcomes did not differ between hyperoxemia and normoxemia groups (Supplementary Table 5).
In the cohort of patients without respiratory infections as primary source (n = 969), hyperoxemia was not significantly associated with 90-day mortality both in the unadjusted and adjusted analysis (Supplementary Table 6A). No significant adjusted association was found between log-transformed continuous PaO2 and 90-day mortality in this group (Supplementary Table 6B). Conversely, the subgroup analysis including patients who had the lung as primary site of infection (n = 663) showed a reduced risk of 90-day mortality in patients with hyperoxemia both in the unadjusted and adjusted analysis (Supplementary Table 7A). This finding was confirmed by the adjusted analysis of the log-transformed continuous PaO2 (Supplementary Table 7B).
Lastly, in the cohort of patients mechanically ventilated (n = 1290) hyperoxemia was not significantly associated with 90-day mortality (Supplementary Table 8A and 8B).
DiscussionThe main finding of this post-hoc analysis of a large RCT is that in septic patients hyperoxemia was not associated with patients’ survival and other relevant clinical outcomes as compared to normoxemia (defined as PaO2 0–48h≤100 mmHg). Similar results were obtained when the cohort was restricted to septic patients admitted after emergency or elective surgery.
A recent meta-analysis of 9 RCTs on higher versus lower oxygenation strategies in an unselected cohort of critically ill patients admitted to intensive care unit found no significant difference in mortality at 90 days between groups (low certainty evidence), but showed a reduced rate of serious adverse events in patients with lower oxygenation strategies.7 Studies specifically evaluating the effect of different levels of PaO2 on mortality of septic patients differ significantly regarding the definition of hyperoxemia and normoxemia, patients’ characteristics, size of the study cohort and design. The HYPERS2S study compared the effect of hyperoxia (defined as Fraction of Inspired Oxygen (FiO2) 1.0) with normoxia (FiO2 set to obtain a target arterial hemoglobin oxygen saturation of 88–95%) during the first 24 h in patients with septic shock.12 The trial was prematurely stopped due to safety reasons because of an observed increase of 28-day mortality in the hyperoxia group as well as serious adverse events such as ICU-acquired weakness and atelectasis. A post-hoc analysis of the same trial found that hyperoxia treatment for 24 h was associated with higher mortality in patients with septic shock.11
A post-hoc analysis of the ICU-ROX trial including mechanically ventilated septic patients found no difference in 90-day mortality or secondary outcomes between patients treated with conservative (lowest FiO2 to keep peripheral oxygen saturation (SpO2) equal or above 91%) vs standard oxygen therapy, but point estimates of treatment effect consistently favored standard oxygen therapy.13 Martin-Fernandez et al. recently published a secondary analysis of a prospective observational trial investigating the effects of hyperoxemia in 454 postsurgical patients with sepsis or septic shock. Patients were stratified based on the PaO2 level at the day of sepsis/septic shock onset (Hyperoxemia: PaO2>100 mmHg, Normoxemia: PaO2≤100 mmHg). Martin-Fernandez et al. found that patients with hyperoxemia had lower 90-day mortality, reduced length of ICU stay and shorter duration of mechanical ventilation in days.5 These findings contrast with our results as we found no survival advantage in patients with hyperoxemia either in the general cohort of septic patients or in the sub-group of surgical patients. On the other hand, we found that septic patients exposed to normoxemia had a shorter duration of mechanical ventilation and a shorter length of stay in ICU.
When patients with lung as primary site of infection were excluded, we found that hyperoxemia was not significantly associated with higher or lower 90-day mortality as well as in patients who underwent mechanical ventilation. Conversely, when we considered patients with lung as primary site, we found an association between hyperoxemia and lower 90-day mortality. A tentative interpretation of this finding may be the enhanced bacterial killing by higher partial pressure of oxygen.5,6 These findings need to be better researched prospectively by future ad hoc studies.
Strengths and limitationsStrengths of the analysis include the high quality of the trial design and of the generated dataset. Moreover, to the best of our knowledge this post-hoc analysis includes the largest cohort of septic patients available. Unlike other studies, we decided to stratify the patients based upon PaO2 values rather than SpO2. This allowed us to avoid the risk of overestimating the effect of eventual brief episodes of rapid and marked desaturation. Lastly, patients’ stratification based on weighted average PaO2 during the first 48 h avoids the possible mis-categorization of patients that could result from the evaluation of a single value. Indeed, as shown in Fig. 1, PaO2 values were similar between the two groups at baseline and differed significantly at 6 and 24 h.
Our study has limitations, mostly inherent to the nature of the study, which is a post-hoc analysis of a previous trial that was not designed to assess the effect of different levels of oxygenation on patient outcomes. So, due to the study design, residual confounding is likely to remain in the adjusted analysis for the primary outcome. Patients’ stratification was based on weighted average values of PaO2 during the first 48 h using the four timepoints collected in the original dataset: weighting on a more granular assessment would improve the categorization in the study groups. Moreover, we could not calculate the possible cumulative effect of exposure to oxygen supplementation over time (after 48 h), as was done in other studies on mechanically ventilated critically ill patients.19 Finally, co-interventions used at the time of patients’ enrollment during the ALBIOS trial may differ from current clinical practice.
ConclusionsIn this post-hoc analysis of a large RCT, hyperoxemia (defined as weighted average PaO2>100 mmHg during the first 48 h) was not associated with improvement in patient survival or other relevant clinical outcomes. Further adequately powered RCTs are needed to draw definitive conclusions on the role of hyperoxemia in septic patients.
DeclarationsEthics approval and consent to participate: Not applicable.
Availability of data and materials: All data generated or analysed during this study are included in this published article and its supplementary information files.
JMo has received consultancy from Active Medical, BV. GG has received payment for lectures from Fisher&Paykel, Getinge, Draeger, Cook, Mundipharma, Pfizer and research grants from Pfizer, MSD, Fisher&Paykel. All other authors declare that they have no competing interests.
Not applicable.