Prior study in healthy subjects has shown a reduction of partial pressure of arterial oxygen (PaO2) by -1.60 kPa/kilometre of altitude gain. However, the association of altitude-related change in PaO2 and altitude-related adverse health effects (ARAHE) in patients with chronic obstructive pulmonary disease (COPD) remain unknown.
ObjectiveTo provide an effect size estimate for the decline in PaO2 with each kilometre of altitude gain and to identify ARAHE in relation to altitude in patients with COPD. www.crd.york.ac.uk/prospero: CRD42020217938.
Data SourcesA systematic search of PubMed and Embase was performed from inception to May 30, 2023.
Study SelectionPeer-reviewed and prospective studies in patients with COPD staying at altitudes >1500 m providing arterial blood gases within the first 3 days at the target altitude.
Data Extraction and SynthesisAggregate data (AD) on study characteristics were extracted, and individual patient data (IPD) were requested. Estimates were pooled using random-effects meta-analysis.
Main Outcome and MeasuresRelative risk estimates and 95 % confidence intervals for the association between PaO2 and altitude in patients with COPD.
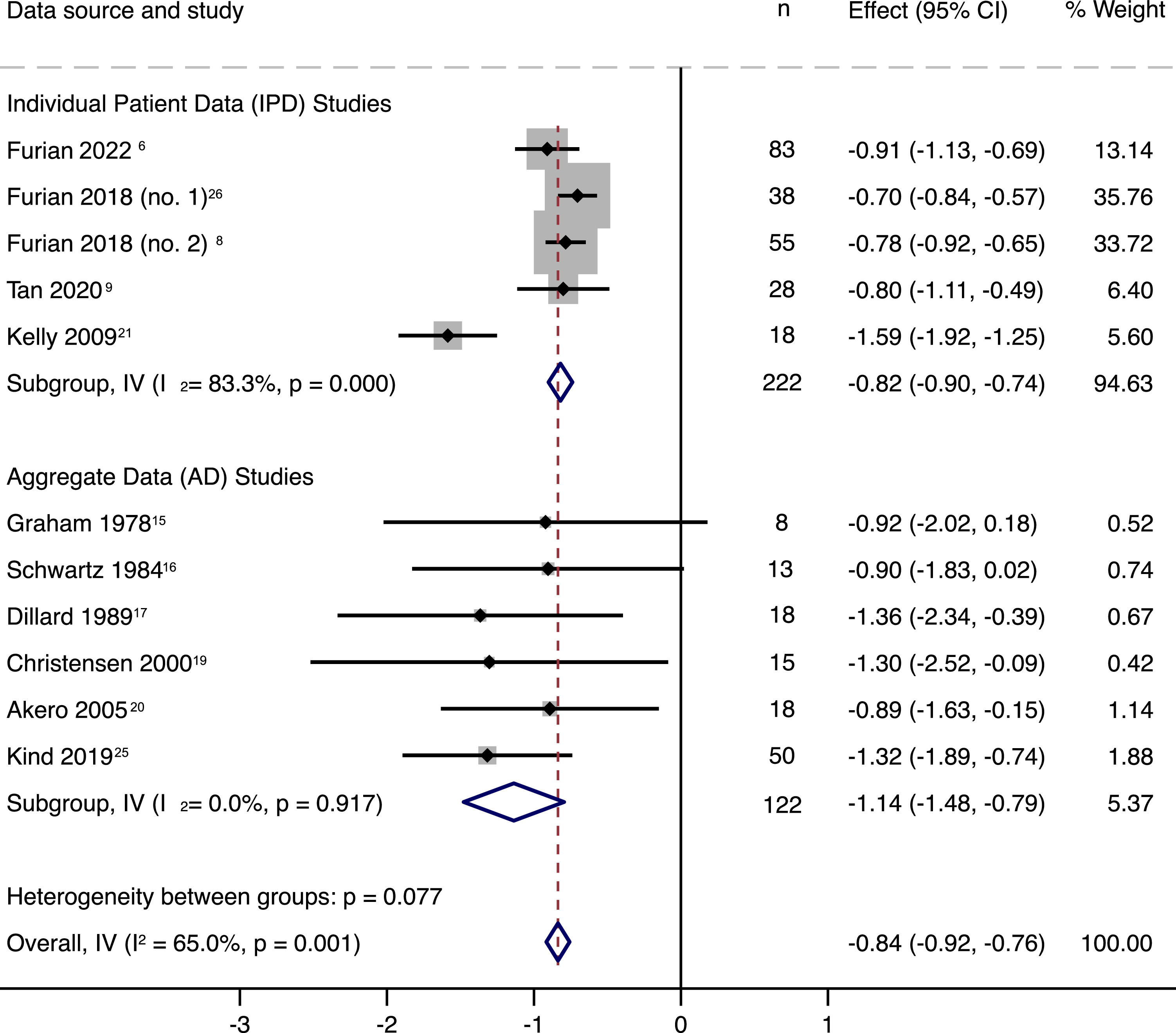
ResultsThirteen studies were included in the AD analysis, of which 6 studies (222 patients, 45.2 % female) provided IPD, thus were included in the quantitative analysis. The estimated effect size of PaO2 was -0.84 kPa [95 %CI, -0.92 to -0.76] per 1000 m of altitude gain (I2=65.0 %, P < 0.001). In multivariable regression analysis, COPD severity, baseline PaO2, age and time spent at altitude were predictors for PaO2 at altitude. Overall, 37.8 % of COPD patients experienced an ARAHE, whereas older age, female sex, COPD severity, baseline PaO2, and target altitude were predictors for the occurrence of ARAHE (area under ROC curve: 0.9275, P < 0.001).
Conclusions and RelevanceThis meta-analysis, providing altitude-related decrease in PaO2 and risk of ARAHE in patients with COPD ascending to altitudes >1500 m, revealed a lower altitude-related decrease in PaO2 in COPD patients compared with healthy. However, these findings might improve patient care and facilitate decisions about initiating preventive measures against hypoxaemia and ARAHE in patients with COPD planning an altitude sojourn or intercontinental flight, i.e. supplemental oxygen or acetazolamide.
With increasing altitude, the atmospheric pressure falls, causing a proportional decrease in partial pressure of inspired oxygen (PiO2) and subsequently reducing partial pressure of arterial oxygen (PaO2).1 Therefore, short-term altitude exposures above 2500 m can cause hypoxaemia, sleep disturbances,2,3 exercise intolerance,4 and acute mountain sickness (AMS) in healthy,5 or altitude-related adverse health effects (ARAHE) in patients.6
Among mountain tourists or air flight passengers, many patients with chronic obstructive pulmonary disease (COPD) are expected to be exposed to hypobaric hypoxia in relation to the global prevalence of 8 – 15 %.7 At low altitudes, patients with COPD commonly develop disease-related mild hypoxaemia and excessive dyspnoea sensation during physical exercise or even at rest. Due to these constraints and previous studies at high altitude in COPD,6,8,9 clinicians should be aware of the current available preventive measures and recommendations for COPD travelling to high altitude.10 Nevertheless, the relationship between absolute altitude, PaO2, and ARAHE in patients with COPD remains unknown. In contrast in healthy subjects, Forrer et al.11 conducted a meta-analysis where they revealed an effect size estimate for the decrease in PaO2 of −1.60 kPa [95 %CI, −1.73 to −1.47] for each 1000 m of altitude gain.
Due to these recent developments and reference PaO2 values in healthy subjects, the purpose of this study was to perform a systematic literature search and meta-analysis to summarise and quantitatively assess the relationship between altitude and PaO2 in patients with COPD. Additionally, we aimed to assess the incidence and type of ARAHE at altitude in COPD. Expected findings will provide clinicians and patients quantitative estimates for the expected PaO2 decline and ARAHE incidence at different altitudes and might facilitate patient's counseling and prescription of preventive measures when planning an altitude sojourn.
MethodsThis systematic literature review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) consensus statement (eTable 1).12 The trial is registered at www.crd.york.ac.uk/prospero: CRD42020217938.
Literature searchA systematic literature search was performed from database inception to May 2023 using MEDLINE and EMBASE databases. Search terms employed for identifying eligible studies are outlined in eMethods. Titles and abstracts of the acquired studies were screened, and full texts were obtained from any that possibly fulfilled the inclusion criteria. Bibliographies of all eligible studies were screened for further appropriate studies.
Data extractionThe eligibility of the studies to be included in the analysis was extracted independently by two investigators using a standardised, pre-piloted form. Based on the data sharing statement, data underlying the results were requested from the corresponding authors. If multiple studies happened to be published from the same cohort, we included only the one with the most complete details. Data extraction discrepancies were resolved through discussion and consensus between two investigators; if needed, a third investigator was consulted. Individual patient data (IPD) underlying the included articles were requested from the corresponding authors.
Study selection criteriaWe aimed to include every peer-reviewed, prospective altitude study and hypobaric chamber study providing arterial blood gas measurements by arterial puncture or catheter at resting conditions in patients with COPD defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).7 We included only studies in patients with COPD travelling to altitudes >1500 m or hypobaric chambers depressurised to altitudes equivalent to >1500 m. Interventional trials that lacked a placebo arm and studies that did not give precise data on the intervention and control groups were excluded. Furthermore, we excluded trials if they were case reports; blood gases were obtained >3 days after arrival at high altitude, and patients were exposed to altitudes >1500 m for >2 days within the last four weeks. Only original articles published in English, French or German were considered.
OutcomesThe primary outcome was the resting PaO2 in patients with COPD travelling to altitudes >1500 m within the first 72 h at target altitude. Secondary outcomes were other parameters of arterial blood gases, such as pH, arterial partial pressure of carbon dioxide (PaCO2), arterial saturation of oxygen (SaO2), haemoglobin, bicarbonate, and finger pulse oximetry (SpO2), respectively, as a function of altitude. To allow further interpretation of the altitude effects, oxygen content (CaO2) was calculated as 1.34 x Hb (g/dl) x SaO2 + PaO2 (kPa) x 0.003. We assessed the reported incidence and type of ARAHE, defined as the occurrence of a health condition requiring medical intervention or premature termination of the study. Additional parameters were retrieved as outlined in the eMethods.
Risk of bias assessmentThe quality of the included studies was evaluated by two independent investigators using the Quality Assessment Tool for observational cohort studies from the National Heart, Lung, and Blood Institute (NIH) outlined in eMethods and eTable 2.13
Statistical analysisAll outcomes were analysed using multiple linear regression adjusting for altitude. The prior linearity assumption was tested for independence, linearity, homoscedasticity of the residuals, and normality of the residuals. The adjusted treatment effect (adjusted absolute difference in means) was then combined across trials using a mixed-effects meta-analysis. For this analysis, the two-stage individual participant data meta-analysis software package was used. Heterogeneity between studies was evaluated using I2 statistics.14 Multivariable logistic regression analysis was applied to develop a prediction model for the occurrence of ARAHE based on altitude and baseline characteristics. All statistical analyses were performed in STATA v18.
ResultsThe literature search of PubMed and Embase provided 177 studies. After assessing all studies according to the pre-specified inclusion and exclusion criteria, 164 studies were excluded for the reasons outlined in eFigure 1. Thirteen original publications providing data on arterial blood gases under hypobaric hypoxic conditions in patients with COPD were included in the aggregated data (AD) end-point analysis.6,8,9,15-24 These articles were published between 1978 and 2020. End-point analysis from individual patient data included six publications from prospective cohort studies in patients with COPD, published between 2009 and 2022. Characteristics of the included studies are summarised in eTable 3. The mean target altitude was 2354 m and ranged from 1650 m to 3100 m.
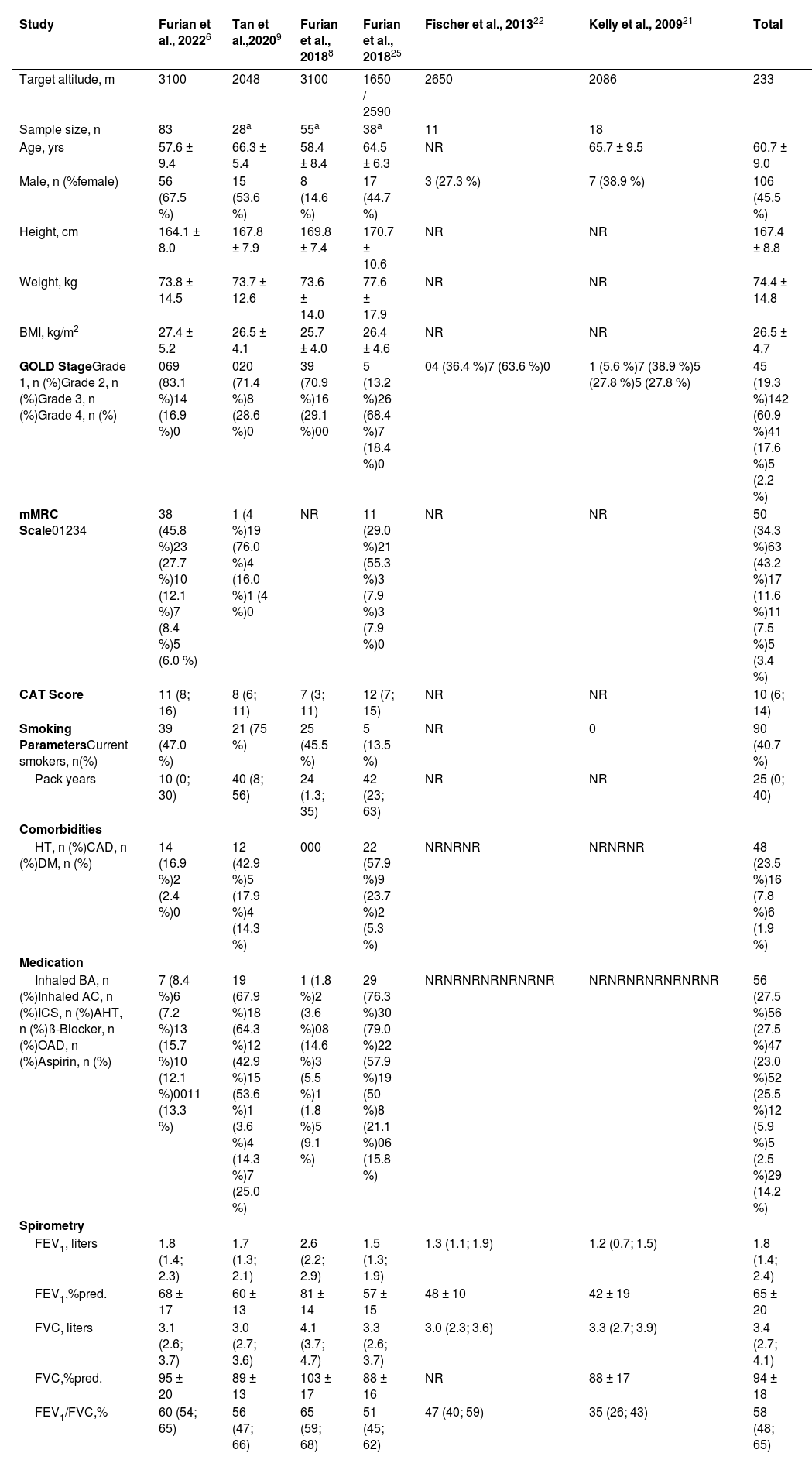
In the AD analysis, 9 out of 13 included studies were high altitude field expeditions conducted in Kyrgyzstan,6,8,24 Switzerland,9,23 Germany,22 New Zealand,21 Norway20, and the United States of America.15 Four additional studies were carried out in a hypobaric hypoxic chamber (simulated altitude: 1650 m to 3048 m) in Norway19 and the United States of America.16-18 Data analysis from IPD included 233 (45.5 % female) patients with COPD receiving either placebo or no medication while being exposed to hypobaric hypoxic conditions (Table 1). The mean ± SD age was 60.7 ± 9.0 years and BMI was 26.5 ± 4.7 kg/m2. Most of the patients with COPD included in the studies had moderate airflow obstruction with COPD GOLD stage 2 and a mean ± SD FEV1 of 65 ± 20%predicted.
Baseline characteristics of studies included in the meta-analysis based on individual patient data (IPD).
| Study | Furian et al., 20226 | Tan et al.,20209 | Furian et al., 20188 | Furian et al., 201825 | Fischer et al., 201322 | Kelly et al., 200921 | Total |
|---|---|---|---|---|---|---|---|
| Target altitude, m | 3100 | 2048 | 3100 | 1650 / 2590 | 2650 | 2086 | 233 |
| Sample size, n | 83 | 28a | 55a | 38a | 11 | 18 | |
| Age, yrs | 57.6 ± 9.4 | 66.3 ± 5.4 | 58.4 ± 8.4 | 64.5 ± 6.3 | NR | 65.7 ± 9.5 | 60.7 ± 9.0 |
| Male, n (%female) | 56 (67.5 %) | 15 (53.6 %) | 8 (14.6 %) | 17 (44.7 %) | 3 (27.3 %) | 7 (38.9 %) | 106 (45.5 %) |
| Height, cm | 164.1 ± 8.0 | 167.8 ± 7.9 | 169.8 ± 7.4 | 170.7 ± 10.6 | NR | NR | 167.4 ± 8.8 |
| Weight, kg | 73.8 ± 14.5 | 73.7 ± 12.6 | 73.6 ± 14.0 | 77.6 ± 17.9 | NR | NR | 74.4 ± 14.8 |
| BMI, kg/m2 | 27.4 ± 5.2 | 26.5 ± 4.1 | 25.7 ± 4.0 | 26.4 ± 4.6 | NR | NR | 26.5 ± 4.7 |
| GOLD StageGrade 1, n (%)Grade 2, n (%)Grade 3, n (%)Grade 4, n (%) | 069 (83.1 %)14 (16.9 %)0 | 020 (71.4 %)8 (28.6 %)0 | 39 (70.9 %)16 (29.1 %)00 | 5 (13.2 %)26 (68.4 %)7 (18.4 %)0 | 04 (36.4 %)7 (63.6 %)0 | 1 (5.6 %)7 (38.9 %)5 (27.8 %)5 (27.8 %) | 45 (19.3 %)142 (60.9 %)41 (17.6 %)5 (2.2 %) |
| mMRC Scale01234 | 38 (45.8 %)23 (27.7 %)10 (12.1 %)7 (8.4 %)5 (6.0 %) | 1 (4 %)19 (76.0 %)4 (16.0 %)1 (4 %)0 | NR | 11 (29.0 %)21 (55.3 %)3 (7.9 %)3 (7.9 %)0 | NR | NR | 50 (34.3 %)63 (43.2 %)17 (11.6 %)11 (7.5 %)5 (3.4 %) |
| CAT Score | 11 (8; 16) | 8 (6; 11) | 7 (3; 11) | 12 (7; 15) | NR | NR | 10 (6; 14) |
| Smoking ParametersCurrent smokers, n(%) | 39 (47.0 %) | 21 (75 %) | 25 (45.5 %) | 5 (13.5 %) | NR | 0 | 90 (40.7 %) |
| Pack years | 10 (0; 30) | 40 (8; 56) | 24 (1.3; 35) | 42 (23; 63) | NR | NR | 25 (0; 40) |
| Comorbidities | |||||||
| HT, n (%)CAD, n (%)DM, n (%) | 14 (16.9 %)2 (2.4 %)0 | 12 (42.9 %)5 (17.9 %)4 (14.3 %) | 000 | 22 (57.9 %)9 (23.7 %)2 (5.3 %) | NRNRNR | NRNRNR | 48 (23.5 %)16 (7.8 %)6 (1.9 %) |
| Medication | |||||||
| Inhaled BA, n (%)Inhaled AC, n (%)ICS, n (%)AHT, n (%)ß-Blocker, n (%)OAD, n (%)Aspirin, n (%) | 7 (8.4 %)6 (7.2 %)13 (15.7 %)10 (12.1 %)0011 (13.3 %) | 19 (67.9 %)18 (64.3 %)12 (42.9 %)15 (53.6 %)1 (3.6 %)4 (14.3 %)7 (25.0 %) | 1 (1.8 %)2 (3.6 %)08 (14.6 %)3 (5.5 %)1 (1.8 %)5 (9.1 %) | 29 (76.3 %)30 (79.0 %)22 (57.9 %)19 (50 %)8 (21.1 %)06 (15.8 %) | NRNRNRNRNRNRNR | NRNRNRNRNRNRNR | 56 (27.5 %)56 (27.5 %)47 (23.0 %)52 (25.5 %)12 (5.9 %)5 (2.5 %)29 (14.2 %) |
| Spirometry | |||||||
| FEV1, liters | 1.8 (1.4; 2.3) | 1.7 (1.3; 2.1) | 2.6 (2.2; 2.9) | 1.5 (1.3; 1.9) | 1.3 (1.1; 1.9) | 1.2 (0.7; 1.5) | 1.8 (1.4; 2.4) |
| FEV1,%pred. | 68 ± 17 | 60 ± 13 | 81 ± 14 | 57 ± 15 | 48 ± 10 | 42 ± 19 | 65 ± 20 |
| FVC, liters | 3.1 (2.6; 3.7) | 3.0 (2.7; 3.6) | 4.1 (3.7; 4.7) | 3.3 (2.6; 3.7) | 3.0 (2.3; 3.6) | 3.3 (2.7; 3.9) | 3.4 (2.7; 4.1) |
| FVC,%pred. | 95 ± 20 | 89 ± 13 | 103 ± 17 | 88 ± 16 | NR | 88 ± 17 | 94 ± 18 |
| FEV1/FVC,% | 60 (54; 65) | 56 (47; 66) | 65 (59; 68) | 51 (45; 62) | 47 (40; 59) | 35 (26; 43) | 58 (48; 65) |
Values are presented in numbers (proportions), mean ± SD or median (interquartile range). AC = Anticholinergics; AHT = Antihypertensives; BA = Beta-Adrenergics; BMI = Body-Mass-Index; CAT = COPD Assessment Test; COPD = Chronic Obstructive Pulmonary Disease; FEV1 = Forced Expiratory Volume in 1 Second; FVC = Forced Vital Capacity; ICS = Inhaled Corticosteroids; IQR = Interquartile Range; mMRC = modified Medical Research Council; NR = Not Reported; OAD = Oral Antidiabetics; OCS = Oral Corticosteroids; SD = Standard Deviation.
The risk of bias assessment was done for all 13 studies and details are outlined in the eResults and eTable 2. Overall, all included trials were considered to have a low or moderate risk of bias.
End-point assessmentsBlood gas analyses at rest were performed either from the radial artery or arterialised ear lobe22 at baseline and high altitude. Measurements of outcome were performed after 20 minutes to 20 h. One study did not report the exact time point of arterial blood gas puncture (range 41 min to 96 min).19 The occurrence of ARAHE at altitude was reported in 9 out of 13 studies. Among others, ARAHE included the diagnosis of AMS by the AMSc questionnaire8,9,25 and/or with the Lake Louise questionnaire,6 and severe hypoxaemia requiring medical intervention, defined by a mean SpO2 of <75 % for >30 min8,25 or <80 % for >30 min.6,9
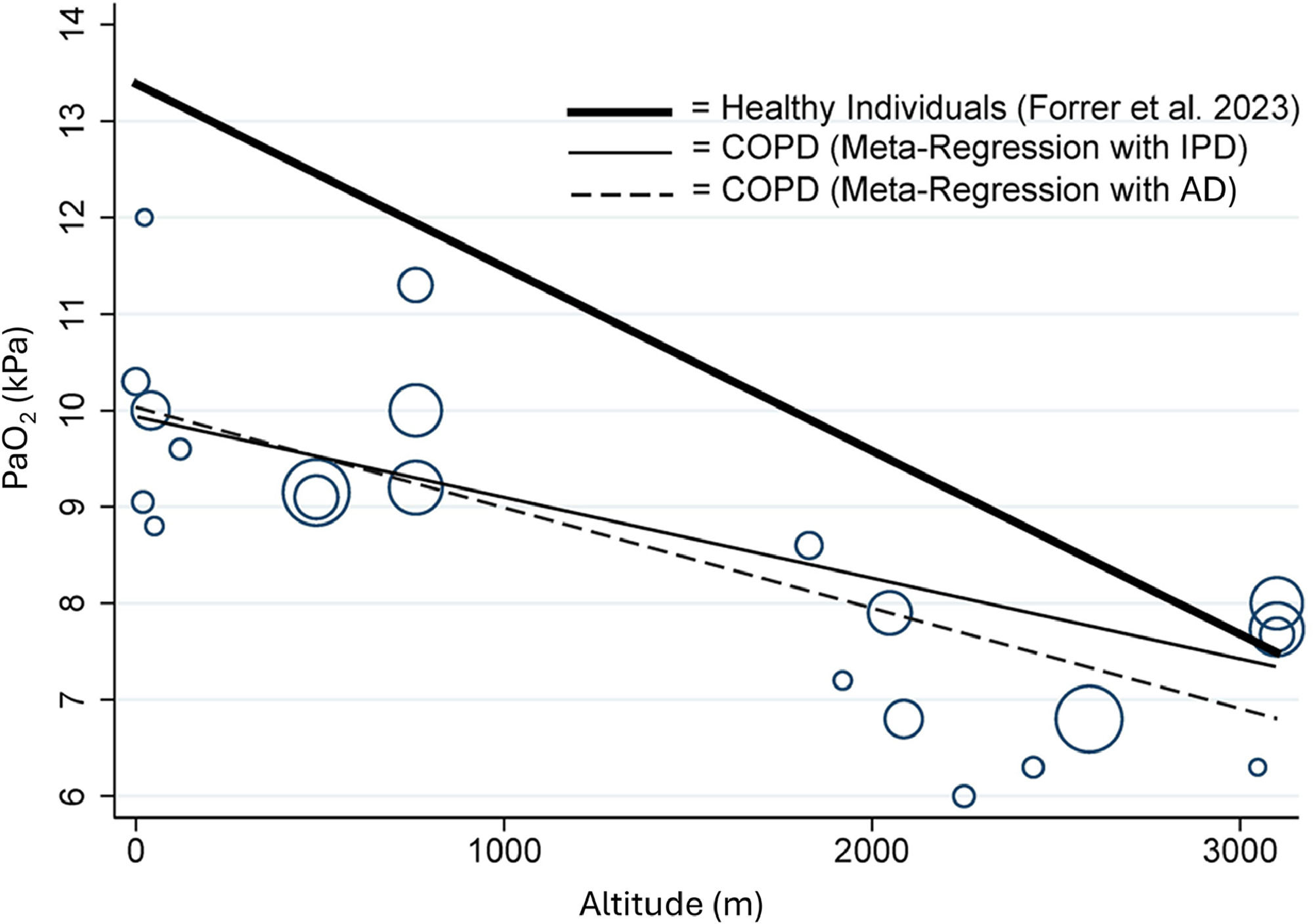
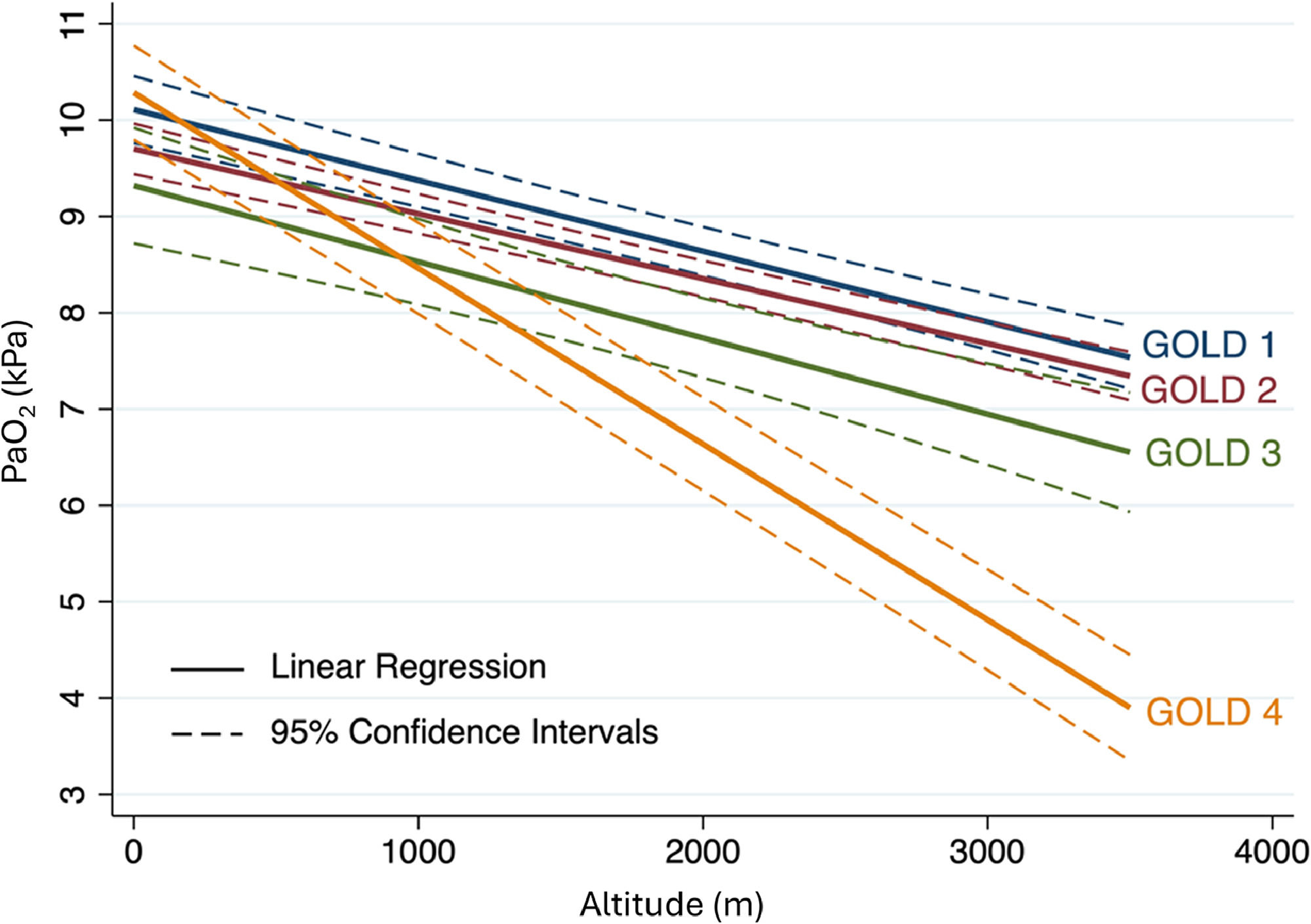
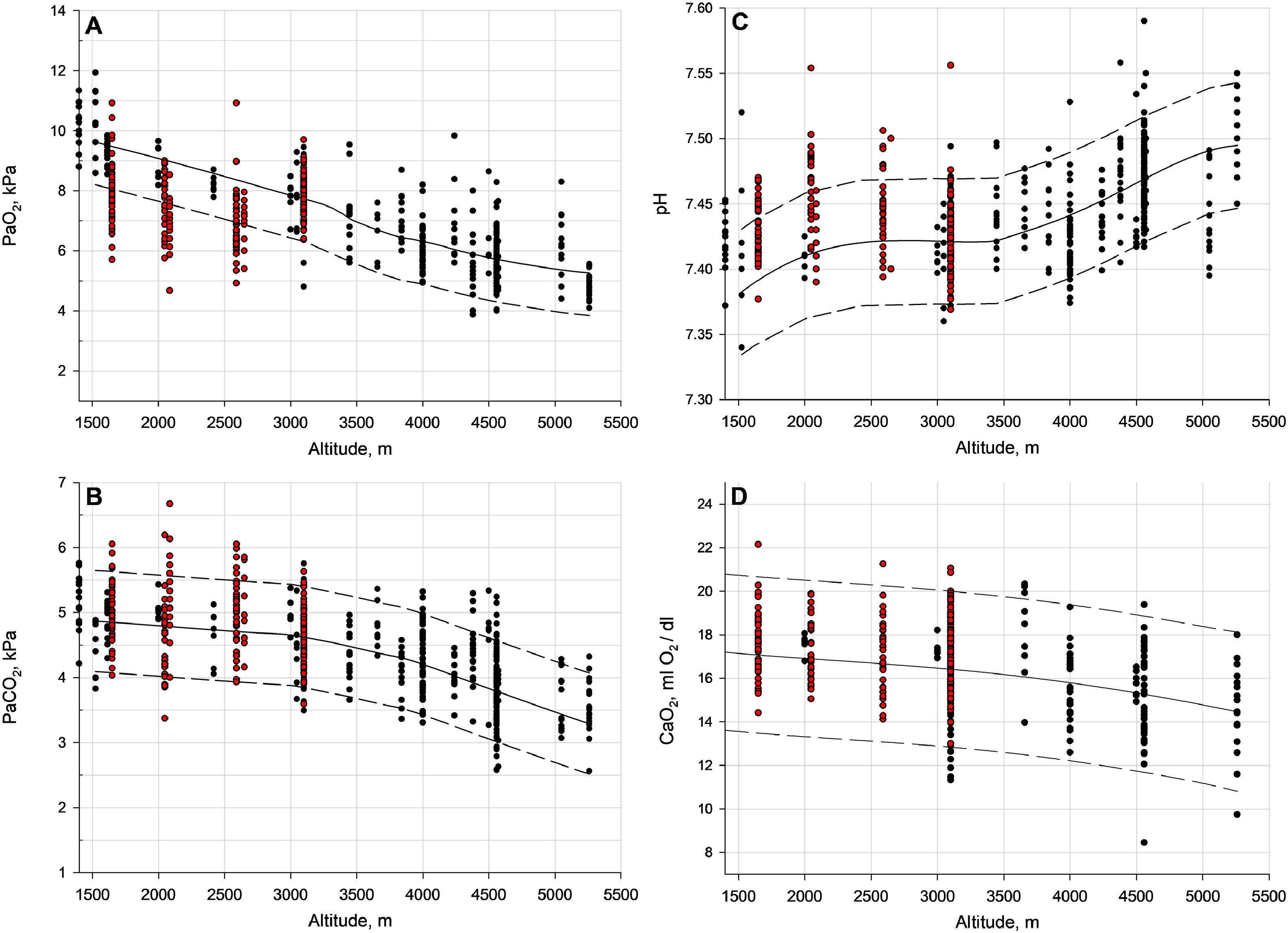
OutcomesThe primary outcome of this meta-analysis, the PaO2, decreased with each 1000 m of altitude gain by 0.84 kPa (95 % CI, 0.76 to 0.92, P < 0.001) (Fig. 1). Linear meta-regression and IPD analysis in 198 COPD patients confirmed this result (Fig. 2, Fig. 3, eTable 4). Moreover, Fig. 2 presents the regression analysis depicting the relationship between altitude gain and the decline of PaO2 in healthy individuals.11 Adjusted regression analysis revealed that COPD severity, baseline PaO2, age and time spent at altitude were influential predictors of PaO2 at altitude (Fig. 3 and eTable 5). Secondary outcome analyses showed an increase in pH and a decrease in SpO2 and PaCO2 with altitude, however, CaO2 remained preserved with altitude (Fig. 4 and eFigure 2).
Forest plot of 11 studies with individual patient data (IPD) and aggregated data (AD) subgroup analysis. Effect of altitude (standardised for 1 km altitude) on partial pressure of arterial oxygen (PaO2). Fischer et al.22 did not assess baseline PaO2, whereas Mehm et al.18 did not provide PaO2 at target altitude, thus were excluded from the analysis.
Relationship between altitude and arterial partial pressure of O2 (PaO2) in patients with COPD, based on aggregated data (AD, dashed line) and individual patient data (IPD, solid line), as well as in healthy individuals (Forrer et al.11 2023, bold line). The size of each bubble is proportional to the standard error (SE) of each of study.
Linear regression analysis for arterial partial pressure of O2 (PaO2) at altitudes by GOLD stage based on individual patient data (IPD). The dataset for individual patient data is displayed using continuous lines to represent the mean partial pressure of arterial oxygen (PaO2) and dashed lines to depict the 95 % confidence interval.
Lower and upper limit of normal for PaO2, PaCO2, pH and CaO2 based on the individual patient data (IPD) in patients with COPD (shown as red dots) from 6 studies, and healthy individuals from 13 studies (Forrer et al.11, shown as black dots). Each panel showcases individual patient data in the form of dots, with the mean represented by a continuous line. The 90 % confidence interval boundaries are illustrated by dashed lines. The lower dashed line signifies the lower limit of normal, while the upper dashed line signifies the upper limit of normal for the specific altitude. The confidence intervals have not been adjusted for potential confounding factors, such as age, gender, BMI, etc. Panel A represents PaO2, Panel B represents PaCO2, Panel C represents pH, and Panel D represents CaO2.
ARAHE requiring medical intervention or relocation to lower altitudes was reported in 88 of 233 (37.8 %) patients travelling to altitudes between 1650 and 3100 m. From the remaining patients able to stay at high altitudes, 25 out of 145 (17.2 %) had PaO2 values <6.6 kPa, theoretically, qualifying for supplemental oxygen therapy as suggested by the British Thoracic Society (BTS) guidelines for pre-flight assessments.26 Exploratory logistic regression analysis revealed that the risk of experiencing an ARAHE when travelling to high altitude with COPD was increased with altitudes higher than 3000 m, age, female sex, COPD GOLD stage, and lower baseline PaO2 (eTable 6). The applied model has an area under the ROC curve of 0.9275, sensitivity of 54 %, specificity of 95 %, positive predictive value of 71 %, and negative predictive value of 90 %. Overall, 87 % of patients were correctly classified by the model described in eTable 6. eTable 7 summarises all incidences and types of reported ARAHEs from studies included in the qualitative analysis (9 out of 13 studies, 334 patients). The main complications at high altitude were severe hypoxaemia and AMS, rarely reported were panic attacks, systemic hypertension, intolerable dyspnoea sensation, COPD exacerbation, and other signs and symptoms from hypoxaemia.
DiscussionThis systematic literature review and meta-analysis in patients with COPD travelling to high altitudes estimates a PaO2 decreased of 0.84 kPa with each 1000 m of altitude gain, whereas lower baseline PaO2, more severe COPD, older age and less time spent at altitude were identified as predictors of worse PaO2. Despite the decrease in PaO2, the CaO2 remained preserved, however, between 1650 and 3100 m, 37.8 % of patients experienced an ARAHE requiring medical intervention or relocation to lower altitudes. Especially older, female patients with very severe COPD and reduced baseline PaO2 were at increased risk of developing an ARAHE. In patients not experiencing an ARAHE, 17.2 % suffered from hypoxaemia exceeding PaO2 values <6.6 kPa, qualifying for cabine supplemental oxygen therapy during airplane travelling.26
The estimated effect size of PaO2 decline in patients with COPD is lower than the reported decline of 1.60 kPa (95 % CI, 1.47 to 1.73) in healthy individuals.11 Underlying mechanisms explaining these differences remain hypothetical, i.e. differences in the PaO2 decline could be related to differences in baseline PaO2 (lower values in COPD), age (COPD were older), hypoxia-related changes in ventilation/perfusion matching, cardiac hypoxic responsiveness, differences in the study setting, timing of measurements or other factors.11
This systematic literature analysis showed that already in 1978, the association between COPD and altitude-induced hypoxaemia was of clinical and scientific interest. During this time period, from 1978 until 2005, a series of sophisticated and pioneering studies were performed in patients with severe COPD (FEV1 of 30 – 49 %predicted), exposing them to conditions equivalent to 2438 m and 3048 m. These studies were designated to assess the ability of COPD patients to tolerate short-term hypoxaemia and whether aircraft travelling with cabine pressurizes equivalent from 1829 m to 2438 m can be recommended to patients with COPD or not.16-20 Based on these studies and results, the hypoxic challenge test (HCT), a fit-to-fly assessment tool for patients with cardiopulmonary limitations, was developed.27 Guidelines currently recommend HCT assessments in COPD patients with resting SpO2 ≤95 %, mMRC score of 3 or greater, or desaturation to <84 % on a six-minute walkt test. 27 However, SpO2 does decrease with age and with higher living altitude even <1500 m;28 moreover, values might be false high due to elevated carboxyhaemoglobin concentration from persistent smoking activity in COPD.29 Due to these and other reasons it has been shown that COPD symptoms and hypoxaemia are not well correlated with FEV1, and even COPD patients with less severe COPD and normal PaO2 at low altitudes might be at risk for hypoxia-related worsening of their disease. Christensen et al.19 showed in 15 patients with mean FEV1 30 ± 12 %predicted and PaO2 values >9.3 kPa that 33 % of these patients suffered from severe hypoxaemia defined by PaO2 <6.6 kPa at 2438 m. These authors emphasized that resting PaO2 >9.3 kPa does not exclude the development of severe hypoxaemia at a moderate altitude equivalent to aircraft travelling and that the current guidelines might insufficiently screen for patients at risk of developing severe hypoxaemia in hypobaric hypoxic conditions. Whether the provided equation from eTable 5 allows a better discrimination of patients requiring in-flight oxygen than the hypoxic challenge test remains to be studied.
Currently, mountain tourism is becoming increasingly popular, in addition to air travel. However, it is important to note that the HCT and other prediction models have not been accurate in determining the altitude tolerance of COPD patients who spend multiple days in hypobaric hypoxic conditions.30 Recent studies in patients with COPD staying at altitudes from 1650 m to 3100 m showed unique disease-related altitude intolerances, assumingly related to disease and altitude-induced hypoxaemia. In a cohort study of 40 patients with moderate-to-severe COPD without daytime hypoxaemia at low altitude (PaO2 ≥7.3 kPa) staying for 2 days at 1650 m and 2590 m each, a total of 9 out of 38 (24 %) patients experienced an ARAHE and reported daytime PaO2 at 2590 m was low with a median (IQR) of 6.8 kPa (6.3; 7.4).25 In another study in 32 patients with moderate-to-severe COPD staying for 2 days and nights at 2048 m, 26 % suffered from ARAHEs, while this was well preserved by 3 L/min nocturnal oxygen (NOT) (ARAHE incidence with NOT: 4 %, P = 0.04).9 Surprisingly, although, NOT prevented ARAHEs, the intervention had no effect on next-morning arterial blood gases (mean difference [95 % CI] NOT vs placebo in PaO2 of −0.1 kPa [−0.4 to 0.1]). This finding indicated that not only the severity but also the duration of persistent hypoxaemia plays an important role in developing hypoxia-related illnesses. This is in accordance with the current literature when comparing well-tolerated hypoxaemia during short-term exposures, similar to aircraft flights, compared to overnight stays at high altitude places.
In one of the first large randomised high altitude trials, including 118 patients with mild-to-moderate COPD, Furian et al.8 investigated whether dexamethasone therapy prevented ARAHE compared to placebo. Compared to patients with moderate-and-severe COPD, patients with mild COPD tolerated the altitude better. This was indicated by comparable ARAHE incidences at 3100 m compared to 2048 m and 2590 m (24 % at 3100 m in patients under placebo). However, comparisons cannot be directly applied, since the ARAHE definition was not identical. Therefore, in the study conducted at 2048 m and 3100 m, severe hypoxaemia was defined as an SpO2 <75 % for >30 min; whereas in the study at 2590 m used an SpO2 <80 % for >30 min. Another explanation of the lower-than-expected incidence of ARAHE at 3100 m in mild COPD might be the normal PaO2 values observed at 760 m (median [IQR], 10.0 kPa [9.1; 10.5]) and the still preserved PaO2 at 3100 m (median [IQR], 8.0 kPa [7.5; 8.4]) in patients in the placebo arm, which might have protected patients from ARAHE progression.
The most recently published randomised clinical trial included 176 patients with moderate-to-severe COPD staying for 2 days and nights at 3100 m.6 The Swiss research group from Bloch et al. investigated whether preventive acetazolamide reduces the incidence of ARAHE in these patients. In contrast to patients with mild COPD (mean FEV1 of 94 %predicted in the placebo arm), moderate-to-severe COPD (mean FEV1 of 63 ± 12 %predicted in the placebo arm) resulted in an ARAHE incidence of 76 %. This high incidence might be partly explained by the less stringent definition of severe hypoxaemia (SpO2 <80 % for >30 min), by the lower baseline PaO2 (9.2 ± 0.1 kPa), and more severe COPD. Indeed, at 3100 m, PaO2 after the first night was 7.7 ± 0.1 kPa and, therefore, lower than in patients with mild COPD. However, direct comparisons of the PaO2 values at 3100 m are not recommended since arterial blood gases were only available in those patients not experiencing an ARAHE on the first night. Therefore, only 29 out of 60 (48 %) patients were available to provide PaO2 values. These competing risks might have induced a considerable bias, and caution is advised for interpreting altitude effects other than ARAHE.
Intriguingly, the literature showed that not only AMS but also other ARAHE occurred, and are therefore of clinical relevance for patients with COPD (eTable 7). Moreover, most ARAHE related to severe hypoxaemia were reported during the night when patients were sleeping. Therefore, during a normal sojourn, these hypoxemic periods remain undetected and asymptomatic. However, the hypoxemic effects on the cardiovascular system in these patients remain to be elucidated.
LimitationsOverall, 13 and 6 studies with 222 patients were available for the AD and IPD analyses. From the 6 studies providing IPD, the study from Fischer et al.22 assessed no baseline PaO2 values and was therefore excluded from the models estimating altitude effects on PaO2. Additionally, 4 of the 6 studies of the IPD analysis were performed by one Swiss group conducting research in Switzerland and Kyrgyzstan; therefore, studies in COPD populations from other regions of the world are desired to improve the generalizability of findings in COPD patients. Moreover, COPD populations studied in Kyrgyzstan seemed to be undertreated compared to current guidelines. We observed competing risks between the occurrence of ARAHEs requiring medical intervention and the feasibility of assessing arterial blood samples in several studies, especially at 3100 m. Therefore, PaO2 estimates might represent patients with less severe COPD and underestimate hypobaric hypoxia's true effects on PaO2. However, it is important to note that such competing risks are unavoidable in high-altitude studies in patients.
ConclusionThis meta-analysis reveals a mean estimate of PaO2 decrease of 0.84 kPa per 1000 m of altitude gain in patients with COPD. Lower baseline PaO2, more severe COPD, older age and less time spent at altitude were predictors for PaO2. Despite preserved CaO2 at altitude, the occurrence of ARAHE was reported in 37.8 % of all patients travelling to high altitudes, and especially older women with progressed COPD and lower baseline PaO2 were at increased risk for experiencing an ARAHE. These novel findings and estimates of PaO2 will allow evidence-based patient's counselling and prescription of preventive measures in COPD planning an intercontinental flight or an altitude sojourn.
Access to data statementAS and MF were provided unrestricted access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Statistical analysis statementTG assumes accountability for the precision of the statistical analysis and the integrity of the results.
Role of funder statementThe sponsor and funding organization were not involved in the design and execution of the study, data collection, management, analysis, or interpretation, nor in the preparation, review, or approval of the manuscript. Furthermore, they did not influence the decision to submit the manuscript for publication.
The authors declare no conflict of interest concerning this work.