Air pollution is a major global environment and health concern. Recent studies have suggested an association between air pollution and COVID-19 mortality and morbidity. In this context, a close association between increased levels of air pollutants such as particulate matter ≤2.5 to 10 µM, ozone and nitrogen dioxide and SARS-CoV-2 infection, hospital admissions and mortality due to COVID 19 has been reported. Air pollutants can make individuals more susceptible to SARS-CoV-2 infection by inducing the expression of proteins such as angiotensin converting enzyme (ACE)2 and transmembrane protease, serine 2 (TMPRSS2) that are required for viral entry into the host cell, while causing impairment in the host defence system by damaging the epithelial barrier, muco-ciliary clearance, inhibiting the antiviral response and causing immune dysregulation. The aim of this review is to report the epidemiological evidence on impact of air pollutants on COVID 19 in an up-to-date manner, as well as to provide insights on in vivo and in vitro mechanisms.
In December 2019, the new coronavirus ‘Severe Acute Respiratory Syndrome Coronavirus 2′ (SARS-CoV-2) was first reported in China (Wuhan, Hubei Province): it still causes coronavirus disease (COVID-19) that can lead to severe pneumonia and lung destruction. SARS-CoV-2 is an enveloped beta coronavirus genetically like SARS-CoV-1.1 COVID-19 became a global health concern in early 2020 and was declared as a pandemic by the World Health Organisation (WHO) on March 11th, 2020. There have been 7,037,007 deaths out of 774,834,251 confirmed cases as of March 3rd, 2024, globally and still counting.2 According to the recent Global Study of Diseases (GBD), Injuries and Risk Factors 2021, all-cause mortality rates in 2020 and 2021 increased in males and females aged 15 years and older comparing to pre-pandemic values in 2019.3
It has been shown that comorbidities are an important risk factor for severe COVID-19.4 In addition, environmental factors such as air pollution have been identified as risk factors for COVID-19 morbidity and mortality.4,5 Emerging evidence suggested an association between environmental factors, particularly air pollution, and COVID-19 cases, in terms of severity and susceptibility of the disease and transmission of the virus.6 As the pandemic tended to disseminate faster in relatively highly air-polluted areas in China and Italy, there was a concern regarding the role of air pollution in the spreading of the infection.7,8 Indeed, studies found a positive association of air pollution with SARS-CoV-2 infection, COVID-19 mortality, and morbidity.7-11 In particular, increased levels of particulate matter ≤ 2.5µM (PM2.5) were associated with increased risk for COVID-19 mortality,12 intensive care unit (ICU) admission13 and hospitalization.10
It is important to remind the attention of major scientific societies and organizations to this topic, as demonstrated by a joint workshop report of the European Respiratory Society (ERS), the International Society of Environmental Epidemiology (ISEE), the Health Effect Institute (HEI) and the World Health Organization (WHO), published in 2021.14 In such a report, the potential role of air pollution in worsening of health impacts of COVID-19, and the influence of the pandemic on air pollution levels in Europe were explored, as well as the major lessons learned to chart a healthy post-pandemic course were outlined.
Furthermore, studies reported that SARS-CoV-2 may exist on ambient PM.15,16 Nevertheless, it is not clear whether this plays a role in the dissemination of SARS-CoV-2 infection since the viability and infective properties of the virus were not investigated. Mechanistic studies suggested that air pollutants can induce cell entry of SARS-CoV-2 and that they can modulate the cellular response to the virus.17-19 Indeed, intratracheal instillation of PM induced expression of ACE-2 and TMPRSS2 in mice lungs,18 while wood smoke increased SARS-CoV-2 titres in human nasal epithelial cells, in vitro.19
This narrative review will focus on epidemiological, in vivo, and in vitro mechanistic data and will provide an updated state-of-the-art presenting the impact of air pollution on mortality and morbidity due to COVID-19.
This paper is the seventh of the Series on “Air pollution and health”.20-25
Literature searchThis manuscript contains epidemiologic and mechanistic studies published in the form of research papers, reviews, and commentaries related to the impact of air pollution on COVID-19 mortality and morbidity. Searches were carried out using PubMed and Google Scholar databases as primary sources, and WHO and Centre for disease control and prevention (CDC) websites as secondary sources using the following search terms “air pollution” or “meteorological parameters”, AND “COVID-19” or “SARS-CoV-2”. Although there was no time restriction for the literature range, almost all the selected references covered the years 2020 to 2024 due to the period of the COVID 19 Pandemic.
Air pollution induces mortality and morbidity due to COVID-19: epidemiological evidenceAir pollution is a major global problem and poses one of the most serious threats to human health; it has been estimated that air pollution annually causes over 7 million premature deaths worldwide.26 According to WHO, about 99% of the world population breathes polluted air, with lower- and middle-income countries most affected by this environmental risk.27 Air pollution is associated with cardiopulmonary mortality and morbidity, and there is a close interaction between air pollution and climate change-related factors, such as increased temperatures, wildfires, and desert dust storms.28
Studies of SARS-CoV-2 have suggested a close link of air pollution with morbidity and mortality due to COVID-19. Most of these studies were from countries such as China, Italy, and the USA that were also severely affected by COVID-19. At the beginning of the pandemic, the outbreaks occurred mostly in the Northern part of Italy, especially in the Po Valley cities of Lodi, Cremona, and Bergamo, which were among the 5 most polluted cities of Italy due to industrialization.29 Moreover, this valley is surrounded by the Alps, causing air trapping due to weak winds. Higher PM2.5 concentrations were correlated with higher all-cause mortality ratios compared with less polluted cities.17 Furthermore, the highest viral lethality was found in other most polluted Italian regions, such as Lombardy, and Emilia Romagna.30
In a study of data from 120 cities in China, Zhu et al. reported that a 10 μg/m3 increase in PM2.5, PM10, nitrogen dioxide (NO2), and ozone (O3) was associated with 2.24%, 1.76%, 6.94%, and 4.76% increases in daily confirmed COVID-19 cases, respectively.8 Similarly, increases in PM2.5 and PM10 augmented case fatality rates by 0.24% and 0.26%, respectively.31 Research conducted in Wuhan and XiaoGan also found a correlation of PM2.5 and NO2 with COVID-19 incidence.32
Similarly, a study from the US showed that an increase of only 1μg/m3 in PM2.5 was associated with an increase of 11 % in deaths due to COVID-19.12 Furthermore, Xu et al. collected data from 806 counties (out of 3143) in the US for 4 months and found that every 10 μg/m3 increment in PM2.5 and O3 levels led to an increase of 9.41% and 2.42%, in mean daily confirmed cases, respectively.33 Adhikari and Yin34 also demonstrated a positive correlation between daily O3 concentrations and new confirmed COVID-19 cases suggesting that air pollutants may have an influence on COVID-19 transmission. In their study investigating the geographical character of the early SARS-CoV-2 infection spread, Pansini and Fornaca reported a positive correlation both for high SARS-CoV-2 infection and COVID-19 mortality rates with increased PM2.5 and NO2 levels in severely affected countries including China, United States of America, Italy, and United Kingdom.35 In a similar fashion, the highest incidence and case-fatality rates were recorded around London and Midlands, zones with the highest air pollution levels observed in the UK.36 Furthermore, a significant correlation between COVID-19 cases and annual average concentration of PM2.5, PM10, SO2, and O3 was observed, together with increased COVID-19 mortality associated with annual averages of these pollutants, in Poland.37 In Istanbul, Turkey, a moderate correlation between the COVID-19 mortality rate per 100 000 population and PM10, SO2, and NO2 was found.11 In Tehran, Iran, there was a positive association between air pollution (in terms of PM2.5, PM10, O3) and COVID-19 hospitalisation/mortality, particularly in the warm season.38 Moreover, an interesting case study from Israel strongly suggested the correlation of population density and city/town total population with COVID-19 morbidity, which may indirectly be attributable to air pollution.39 These studies are summarised in Table 1.
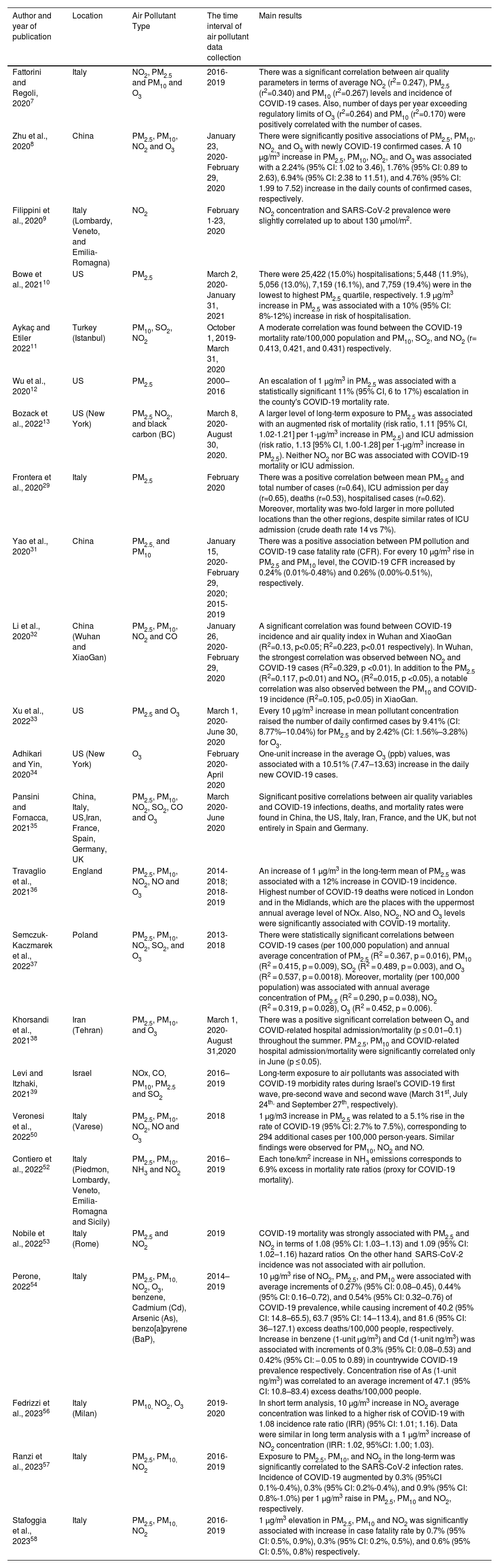
Epidemiological studies investigating the association of air pollution and COVID-19 incidence, hospitalisation, and mortality.
| Author and year of publication | Location | Air Pollutant Type | The time interval of air pollutant data collection | Main results |
|---|---|---|---|---|
| Fattorini and Regoli, 20207 | Italy | NO2, PM2.5 and PM10 and O3 | 2016-2019 | There was a significant correlation between air quality parameters in terms of average NO2 (r2= 0.247), PM2.5 (r2=0.340) and PM10 (r2=0.267) levels and incidence of COVID-19 cases. Also, number of days per year exceeding regulatory limits of O3 (r2=0.264) and PM10 (r2=0.170) were positively correlated with the number of cases. |
| Zhu et al., 20208 | China | PM2.5, PM10, NO2 and O3 | January 23, 2020-February 29, 2020 | There were significantly positive associations of PM2.5, PM10, NO2, and O3 with newly COVID-19 confirmed cases. A 10 μg/m3 increase in PM2.5, PM10, NO2, and O3 was associated with a 2.24% (95% CI: 1.02 to 3.46), 1.76% (95% CI: 0.89 to 2.63), 6.94% (95% CI: 2.38 to 11.51), and 4.76% (95% CI: 1.99 to 7.52) increase in the daily counts of confirmed cases, respectively. |
| Filippini et al., 20209 | Italy (Lombardy, Veneto, and Emilia-Romagna) | NO2 | February 1-23, 2020 | NO2 concentration and SARS-CoV-2 prevalence were slightly correlated up to about 130 μmol/m2. |
| Bowe et al., 202110 | US | PM2.5 | March 2, 2020-January 31, 2021 | There were 25,422 (15.0%) hospitalisations; 5,448 (11.9%), 5,056 (13.0%), 7,159 (16.1%), and 7,759 (19.4%) were in the lowest to highest PM2.5 quartile, respectively. 1.9 µg/m3 increase in PM2.5 was associated with a 10% (95% CI: 8%-12%) increase in risk of hospitalisation. |
| Aykaç and Etiler 202211 | Turkey (Istanbul) | PM10, SO2, NO2 | October 1, 2019- March 31, 2020 | A moderate correlation was found between the COVID-19 mortality rate/100,000 population and PM10, SO2, and NO2 (r= 0.413, 0.421, and 0.431) respectively. |
| Wu et al., 202012 | US | PM2.5 | 2000–2016 | An escalation of 1 μg/m3 in PM2.5 was associated with a statistically significant 11% (95% CI, 6 to 17%) escalation in the county's COVID-19 mortality rate. |
| Bozack et al., 202213 | US (New York) | PM2.5 NO2, and black carbon (BC) | March 8, 2020- August 30, 2020. | A larger level of long-term exposure to PM2.5 was associated with an augmented risk of mortality (risk ratio, 1.11 [95% CI, 1.02-1.21] per 1-μg/m3 increase in PM2.5) and ICU admission (risk ratio, 1.13 [95% CI, 1.00-1.28] per 1-μg/m3 increase in PM2.5). Neither NO2 nor BC was associated with COVID-19 mortality or ICU admission. |
| Frontera et al., 202029 | Italy | PM2.5 | February 2020 | There was a positive correlation between mean PM2.5 and total number of cases (r=0.64), ICU admission per day (r=0.65), deaths (r=0.53), hospitalised cases (r=0.62). Moreover, mortality was two-fold larger in more polluted locations than the other regions, despite similar rates of ICU admission (crude death rate 14 vs 7%). |
| Yao et al., 202031 | China | PM2.5, and PM10 | January 15, 2020- February 29, 2020; 2015-2019 | There was a positive association between PM pollution and COVID-19 case fatality rate (CFR). For every 10 μg/m3 rise in PM2.5 and PM10 level, the COVID-19 CFR increased by 0.24% (0.01%-0.48%) and 0.26% (0.00%-0.51%), respectively. |
| Li et al., 202032 | China (Wuhan and XiaoGan) | PM2.5, PM10, NO2 and CO | January 26, 2020-February 29, 2020 | A significant correlation was found between COVID-19 incidence and air quality index in Wuhan and XiaoGan (R2=0.13, p<0.05; R2=0.223, p<0.01 respectively). In Wuhan, the strongest correlation was observed between NO2 and COVID-19 cases (R2=0.329, p <0.01). In addition to the PM2.5 (R2=0.117, p<0.01) and NO2 (R2=0.015, p <0.05), a notable correlation was also observed between the PM10 and COVID-19 incidence (R2=0.105, p<0.05) in XiaoGan. |
| Xu et al., 202233 | US | PM2.5 and O3 | March 1, 2020- June 30, 2020 | Every 10 μg/m3 increase in mean pollutant concentration raised the number of daily confirmed cases by 9.41% (CI: 8.77%–10.04%) for PM2.5 and by 2.42% (CI: 1.56%–3.28%) for O3. |
| Adhikari and Yin, 202034 | US (New York) | O3 | February 2020- April 2020 | One-unit increase in the average O3 (ppb) values, was associated with a 10.51% (7.47–13.63) increase in the daily new COVID-19 cases. |
| Pansini and Fornacca, 202135 | China, Italy, US,Iran, France, Spain, Germany, UK | PM2.5, PM10, NO2, SO2, CO and O3 | March 2020- June 2020 | Significant positive correlations between air quality variables and COVID-19 infections, deaths, and mortality rates were found in China, the US, Italy, Iran, France, and the UK, but not entirely in Spain and Germany. |
| Travaglio et al., 202136 | England | PM2.5, PM10, NO2, NO and O3 | 2014-2018; 2018-2019 | An increase of 1 μg/m3 in the long-term mean of PM2.5 was associated with a 12% increase in COVID-19 incidence. Highest number of COVID-19 deaths were noticed in London and in the Midlands, which are the places with the uppermost annual average level of NOx. Also, NO2, NO and O3 levels were significantly associated with COVID-19 mortality. |
| Semczuk-Kaczmarek et al., 202237 | Poland | PM2.5, PM10, NO2, SO2, and O3 | 2013- 2018 | There were statistically significant correlations between COVID-19 cases (per 100,000 population) and annual average concentration of PM2.5 (R2 = 0.367, p = 0.016), PM10 (R2 = 0.415, p = 0.009), SO2 (R2 = 0.489, p = 0.003), and O3 (R2 = 0.537, p = 0.0018). Moreover, mortality (per 100,000 population) was associated with annual average concentration of PM2.5 (R2 = 0.290, p = 0.038), NO2 (R2 = 0.319, p = 0.028), O3 (R2 = 0.452, p = 0.006). |
| Khorsandi et al., 202138 | Iran (Tehran) | PM2.5, PM10, and O3 | March 1, 2020- August 31,2020 | There was a positive significant correlation between O3 and COVID-related hospital admission/mortality (p ≤ 0.01–0.1) throughout the summer. PM.2.5, PM10 and COVID-related hospital admission/mortality were significantly correlated only in June (p ≤ 0.05). |
| Levi and Itzhaki, 202139 | Israel | NOx, CO, PM10, PM2.5 and SO2 | 2016–2019 | Long-term exposure to air pollutants was associated with COVID-19 morbidity rates during Israel's COVID-19 first wave, pre-second wave and second wave (March 31st, July 24th, and September 27th, respectively). |
| Veronesi et al., 202250 | Italy (Varese) | PM2.5, PM10, NO2, NO and O3 | 2018 | 1 µg/m3 increase in PM2.5 was related to a 5.1% rise in the rate of COVID-19 (95% CI: 2.7% to 7.5%), corresponding to 294 additional cases per 100,000 person-years. Similar findings were observed for PM10, NO2 and NO. |
| Contiero et al., 202252 | Italy (Piedmon, Lombardy, Veneto, Emilia-Romagna and Sicily) | PM2.5, PM10, NH3 and NO2 | 2016–2019 | Each tone/km2 increase in NH3 emissions corresponds to 6.9% excess in mortality rate ratios (proxy for COVID-19 mortality). |
| Nobile et al., 202253 | Italy (Rome) | PM2.5 and NO2 | 2019 | COVID-19 mortality was strongly associated with PM2.5 and NO2 in terms of 1.08 (95% CI: 1.03–1.13) and 1.09 (95% CI: 1.02–1.16) hazard ratios. On the other hand, SARS-CoV-2 incidence was not associated with air pollution. |
| Perone, 202254 | Italy | PM2.5, PM10, NO2, O3, benzene, Cadmium (Cd), Arsenic (As), benzo[a]pyrene (BaP), | 2014–2019 | 10 μg/m3 rise of NO2, PM2.5, and PM10 were associated with average increments of 0.27% (95% CI: 0.08–0.45), 0.44% (95% CI: 0.16–0.72), and 0.54% (95% CI: 0.32–0.76) of COVID-19 prevalence, while causing increment of 40.2 (95% CI: 14.8–65.5), 63.7 (95% CI: 14–113.4), and 81.6 (95% CI: 36–127.1) excess deaths/100,000 people, respectively. Increase in benzene (1-unit µg/m3) and Cd (1-unit ng/m3) was associated with increments of 0.3% (95% CI: 0.08–0.53) and 0.42% (95% CI: − 0.05 to 0.89) in countrywide COVID-19 prevalence respectively. Concentration rise of As (1-unit ng/m3) was correlated to an average increment of 47.1 (95% CI: 10.8–83.4) excess deaths/100,000 people. |
| Fedrizzi et al., 202356 | Italy (Milan) | PM10, NO2, O3 | 2019-2020 | In short term analysis, 10 μg/m3 increase in NO2 average concentration was linked to a higher risk of COVID-19 with 1.08 incidence rate ratio (IRR) (95% CI: 1.01; 1.16). Data were similar in long term analysis with a 1 μg/m3 increase of NO2 concentration (IRR: 1.02, 95%CI: 1.00; 1.03). |
| Ranzi et al., 202357 | Italy | PM2.5, PM10, NO2 | 2016-2019 | Exposure to PM2.5, PM10, and NO2 in the long-term was significantly correlated to the SARS-CoV-2 infection rates. Incidence of COVID-19 augmented by 0.3% (95%CI 0.1%-0.4%), 0.3% (95% CI: 0.2%-0.4%), and 0.9% (95% CI: 0.8%-1.0%) per 1 μg/m3 raise in PM2.5, PM10 and NO2, respectively. |
| Stafoggia et al., 202358 | Italy | PM2.5, PM10, NO2 | 2016-2019 | 1 μg/m3 elevation in PM2.5, PM10 and NO2 was significantly associated with increase in case fatality rate by 0.7% (95% CI: 0.5%, 0.9%), 0.3% (95% CI: 0.2%, 0.5%), and 0.6% (95% CI: 0.5%, 0.8%) respectively. |
Parameters related to climate change may have an impact on the spread of SARS-CoV-2 by both affecting viral diffusion / viral survival outside of the host and the immunity of the population.40 The impact of temperature and humidity has been relatively well studied. For instance, according to several studies from China, low temperature, low humidity, and mild diurnal temperature range facilitate SARS-CoV-2 transmission.41-43 Yao et al. reported that COVID-19 transmission is negatively affected by higher temperature and lower relative humidity.44 In an extensive study, which was conducted in 3739 locations worldwide, between December 12, 2019, and April 22, 2020, the authors found a negative relationship between temperatures above 25°C and transmission rate of the virus, suggesting that lower temperature can induce SARS-CoV-2 infection.45 Furthermore, there was a weaker association with diurnal temperature, while a U-shaped relationship with outdoor ultraviolet (UV) exposure was noted. Together these findings suggest that changes in meteorological parameters may impact the viability of SARS-CoV-2 and transmission of the virus.
There is a lack of studies investigating the association between viral transmission and other meteorological parameters, such as wind speed and direction, and atmospheric pressure. However, Zhou et al. (2022) investigated the effect of daily average wind speed in four different states in the US. The number of confirmed COVID-19 cases in New York, California, and Texas was negatively correlated with daily average wind speed, while there was no correlation for Florida, and the authors concluded that low wind speed facilitated viral spread.46 In another study, a mild positive association of wind speed, precipitation, and air pressure with SARS-CoV-2 infection was found.45 The study by Adhikari and Yin34 showed that meteorological factors, such as wind speed, temperature, precipitation, and relative humidity, were associated with new COVID-19 cases.
Few studies investigated the mechanisms underlying the impact of meteorological factors on SARS-CoV-2 infection. Environmental conditions may affect the stability of the virus in nasal mucus and sputum. It was found that SARS-CoV-2 is more stable at low-temperature and low-humidity conditions and that warmer temperatures and higher humidity shortened its half-life.47 It has been suggested that single-stranded RNA viruses like SARS-CoV-2 are susceptible to UV inactivation6 and that a reduction in UV penetration may facilitate virus persistence in the ambient atmosphere. Furthermore, a decrease in UV is thought to reduce the synthesis of vitamin D, which has an antioxidant effect and has a positive role in a proper defence system against SARS-CoV-248. Finally, cold, and dry weather is also reported to induce susceptibility of the host immunity to the virus.49 These studies are summarised in Table 2.
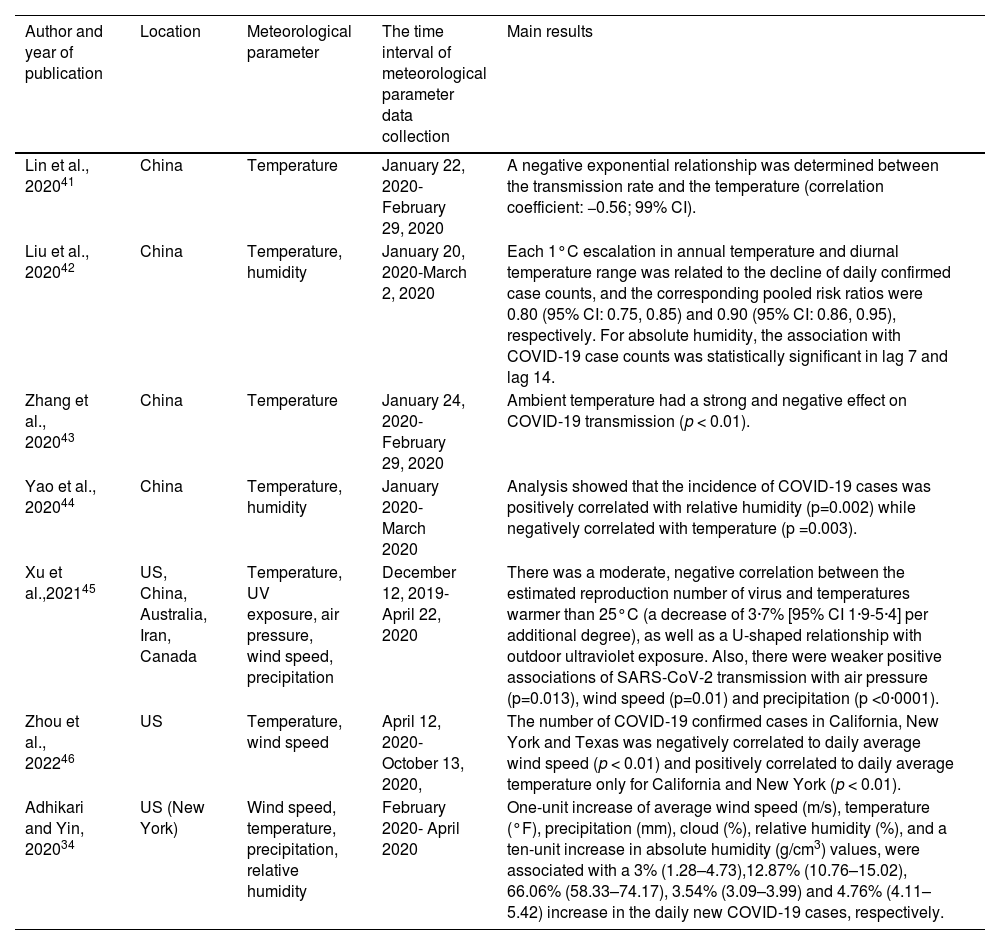
Epidemiological studies investigating the association of meteorological parameters with COVID-19 incidence, hospitalisation, and mortality.
| Author and year of publication | Location | Meteorological parameter | The time interval of meteorological parameter data collection | Main results |
|---|---|---|---|---|
| Lin et al., 202041 | China | Temperature | January 22, 2020- February 29, 2020 | A negative exponential relationship was determined between the transmission rate and the temperature (correlation coefficient: −0.56; 99% CI). |
| Liu et al., 202042 | China | Temperature, humidity | January 20, 2020-March 2, 2020 | Each 1°C escalation in annual temperature and diurnal temperature range was related to the decline of daily confirmed case counts, and the corresponding pooled risk ratios were 0.80 (95% CI: 0.75, 0.85) and 0.90 (95% CI: 0.86, 0.95), respectively. For absolute humidity, the association with COVID-19 case counts was statistically significant in lag 7 and lag 14. |
| Zhang et al., 202043 | China | Temperature | January 24, 2020- February 29, 2020 | Ambient temperature had a strong and negative effect on COVID-19 transmission (p < 0.01). |
| Yao et al., 202044 | China | Temperature, humidity | January 2020- March 2020 | Analysis showed that the incidence of COVID-19 cases was positively correlated with relative humidity (p=0.002) while negatively correlated with temperature (p =0.003). |
| Xu et al.,202145 | US, China, Australia, Iran, Canada | Temperature, UV exposure, air pressure, wind speed, precipitation | December 12, 2019-April 22, 2020 | There was a moderate, negative correlation between the estimated reproduction number of virus and temperatures warmer than 25°C (a decrease of 3·7% [95% CI 1·9-5·4] per additional degree), as well as a U-shaped relationship with outdoor ultraviolet exposure. Also, there were weaker positive associations of SARS-CoV-2 transmission with air pressure (p=0.013), wind speed (p=0.01) and precipitation (p <0·0001). |
| Zhou et al., 202246 | US | Temperature, wind speed | April 12, 2020- October 13, 2020, | The number of COVID-19 confirmed cases in California, New York and Texas was negatively correlated to daily average wind speed (p < 0.01) and positively correlated to daily average temperature only for California and New York (p < 0.01). |
| Adhikari and Yin, 202034 | US (New York) | Wind speed, temperature, precipitation, relative humidity | February 2020- April 2020 | One-unit increase of average wind speed (m/s), temperature (°F), precipitation (mm), cloud (%), relative humidity (%), and a ten-unit increase in absolute humidity (g/cm3) values, were associated with a 3% (1.28–4.73),12.87% (10.76–15.02), 66.06% (58.33–74.17), 3.54% (3.09–3.99) and 4.76% (4.11–5.42) increase in the daily new COVID-19 cases, respectively. |
In summary, epidemiological studies conducted in different countries and regions have demonstrated that there is clear evidence suggesting an association between air pollution and an increased risk for COVID-19 morbidity and mortality worldwide. Thus, an improvement in the air quality can be expected to have a positive impact on the outcomes of COVID 19. On the other hand, studies on the role of meteorological parameters on COVID 19 are lacking clear evidence. However, a limited number of studies have suggested that lower temperatures can facilitate the spread of SARS-CoV-2 that could lead to increased number of COVID 19 cases.
An Italian epidemiological perspective on COVID-19 and air pollutionItaly, one of the first Western countries hit by the pandemic, has been the site of many recent epidemiological contributions (Table 1).
Veronesi et al., 50 in a prospective study in the city of Varese, were able to link citizens by residential address to 2018 average annual exposure to outdoor concentrations of air pollutants modelled using FARM, a chemical transport model. Citizens were further linked to regional datasets for COVID-19 case ascertainment. The authors found that PM2.5 was associated with a 5.1% (95% confidence interval [CI]= 2.7% to 7.5%) increase in the rate of COVID-19, corresponding to 294 additional cases per 100,000 person-years. Similar findings were observed for PM10, NO2, and NO.
Tateo et al.,51 in a study on environmental factors influencing SARS-CoV-2 spreading in North Italy, examined eight administrative areas (altogether 26 million inhabitants) and were able to demonstrate that PM matched the growth of hospitalisations in areas with low chronic particulate pollution, whilst fewer hospitalisations strongly corresponded to the higher temperature and solar radiation. They hypothesised that solar radiation alone and combined with high temperature exert an anti-SARS-CoV-2 effect, via both direct inactivation of virions and stimulation of vitamin D synthesis, improving function of the immune system.
Contiero et al.52 performed an ecological study of five Italian Regions (Piedmont, Lombardy, Veneto, Emilia-Romagna and Sicily), linking all-cause mortality by province (administrative entities within regions) to data on atmospheric concentrations of PM (PM2.5 and PM10) and ammonia (NH3), which are mainly produced by agricultural activities, documenting a 6.9% excess in a proxy for COVID-19 mortality for each tonne/km2 increase in NH3 emissions.
Nobile et al.53 reported that long-term exposure to air pollution (PM2.5 and NO2) was associated with COVID-19 mortality, but not with SARS-CoV-2 incidence, in a large observational population-based cohort of >1.5 million subjects in Rome.
Perone54 assessed air quality in 107 Italian provinces in the 2014-2019 period, and the association between exposure to nine outdoor air pollutants and COVID-19’s spread and related mortality in the same areas. The results showed that long-term exposure to NO2, PM2.5, PM10, benzene, benzo[a]pyrene (BaP), and cadmium (Cd) was positively and significantly correlated with the spread of COVID-19; in addition, long-term exposure to NO2, O3, PM2.5, PM10, and arsenic (As) was positively and significantly correlated with COVID-19 related mortality. In detail, PM and Cd showed the most adverse effect on COVID-19 prevalence, while PM and As showed the most relevant impact on excess mortality rate.
Falco et al.55 explored the possible beneficial effect of green areas in Spain and Italy: for both countries, a statistically significant association between COVID-19 clinical features (contagions, hospitalisations, and deaths) and the extension of public green areas (negative correlation), as well as the annual average concentrations of PM2.5 (positive correlation), clearly emerged.
Fedrizzi et al.,56 investigating a cohort of health care workers in Milan, found that a 10 μg/m3 increase in NO2 average concentration in the four days preceding nasopharyngeal swab for detecting SARS-CoV-2 was associated with a higher risk of testing positive (Incidence Rate Ratio [IRR] = 1.08, 95% CI: 1.01; 1.16). When considering a 1 μg/m3 increase in 2019 annual NO2 average, they observed a higher risk of infection (IRR: 1.02, 95%CI: 1.00; 1.03) and an increased antibody titre (IRR: 2.4%, 95%CI: 1.1; 3.6%).
The large availability of both air pollution and COVID-19 data, and the simplicity to make geographical correlations between them, led to a proliferation of ecological studies relating the levels of pollution in administrative areas to COVID-19 incidence, mortality, or lethality rates. However, the major drawback of these studies is the ecological fallacy that can lead to spurious associations. This has prompted a group of researchers from several epidemiological units, including the National Institute of Health (Istituto Superiore di Sanità) to launch the EpiCovAir study.56,57 Initially, the authors investigated the association between long-term exposure to air pollutants and the incidence of SARS-CoV-2 infections in Italy.57 A satellite-based air pollution exposure model with 1-km2 spatial resolution for entire Italy was applied and 2016-2019 mean population-weighted concentrations of PM10, PM2.5, and NO2 were calculated for each municipality, as estimates of chronic exposures. Individual records of diagnosed SARS-2-CoV-2 infections in Italy from February 2020 to June 2021 reported to the Italian Integrated Surveillance of COVID-19 were used. Updated statistical techniques were applied: principal component analysis (PCA); a mixed longitudinal ecological design with the study units consisting of individual municipalities in Italy; and generalised negative binomial models controlling for several confounders/effect modifiers. Almost 4 million COVID-19 cases in 7,800 municipalities were analysed (total population: 59,589,357 inhabitants). It was found that long-term exposure to PM2.5, PM10, and NO2 was significantly associated with the incidence rates of SARS-CoV-2 infection. In particular, incidence of COVID-19 increased by 0.3% (95%CI: 0.1%-0.4%), 0.3% (0.2%-0.4%), and 0.9% (0.8%-1.0%) per 1 μg/m3 increment in PM2.5, PM10 and NO2, respectively. Associations were higher among elderly subjects and during the second pandemic wave (September 2020-December 2020).
Then, the authors aimed to investigate the association between long-term exposure to air pollutants and mortality among 4 million COVID-19 cases in Italy.58 They used the same COVID population and methods for attributing air pollution exposures and for statistical analysis, adding a generalised propensity score (GPS) approach to an extensive list of area-level covariates to account for major determinants of the spatial distribution of COVID-19 case-fatality rates. Overall, case-fatality rates increased by 0.7% [95% CI: 0.5%, 0.9%], 0.3% (95% CI: 0.2%, 0.5%), and 0.6% (95% CI: 0.5%, 0.8%) per 1μg/m3 increment in PM2.5, PM10, and NO2, respectively. Associations were higher among elderly subjects during the first (February 2020-June 2020) and the third (December 2020-June 2021) pandemic waves. They estimated that ∼8% of COVID-19 deaths were attributable to pollutant levels above the WHO 2021 air quality guidelines.
In summary, the recent epidemiological contributions from Italy have consistently reported a relationship of air pollution exposure with several health outcomes linked to COVID-19.
A Portuguese epidemiological perspective on COVID-19 and air pollutionFewer studies were conducted in Portugal, mostly involving indoor air quality and the effects of lockdown or modelling air pollutants in cities. There is not yet a large epidemiological study to assess the effects of outdoor air pollutants on COVID-19 incidence or mortality.
Indeed, there has been an important nationwide study to evaluate the effects of the 2020 lockdown on air quality.59 This study evaluated air pollution changes across all of Portugal (68 stations) considering all urban, suburban, and rural zones. PM10, PM2.5, NO2, SO2, and ozone were analysed in the pre-, during, and post-lockdown period (January-May 2020) and, for a comparison, also in 2019. NO2 was the most reduced pollutant in 2020, which coincided with decreased traffic. A significant drop (15-71%) in traffic-related NO2 was observed specifically during the lockdown period, 55% for the largest and most populated region in the country. The PM was affected to a lesser degree, with substantial differences found for largely populated areas (Lisbon region ∼ 30%; North region, up to 49%); during lockdown, traffic-related PM dropped 10-70%. PM10 daily limit was exceeded 50% less in 2020, with 80% of exceedances before the lockdown period. SO2 decreased by 35%, due to suspended industrial productions, whereas ozone concentrations slightly (though not significantly) increased (83 vs. 80 µg m-3).
Interestingly, a systematic review of the impact of COVID 19 pandemic on air quality was published by Portuguese authors in 2022.60 A total of 114 studies that quantified the impact of the COVID-19 pandemic on air quality through monitoring were selected from three databases. The authors found that, although using different methodologies, some studies reported a temporary air quality improvement during the lockdown. More studies are still needed, comparing different lockdown, and lifting periods and, in other areas, for a definition of better-targeted policies to reduce air pollution.
Another study aimed to understand the influence of industries (including steelworks, lime factories, and industry of metal waste management and treatment) on the air quality of the urban-industrial area of Seixal (Portugal), during the first period of national lockdown due to COVID-19, whereas local industries kept their normal working schedule.61 The impact of the industries located in the study area on local air quality was identified (namely, the steelworks), confirming the concerns of the local population. The authors deemed that this valuable information is essential to improve future planning and optimize the assessment of particulate matter levels by reference methods.
In summary, the epidemiological studies from Portugal specifically focused on impact of lockdowns on air quality, and it was found that decreases in traffic and industrial activities led to an improvement in the air quality. It would be useful to realize a large epidemiological study to assess the effects of outdoor air pollutants on COVID-19 incidence or mortality.
Effects of air pollution on COVID-19: putative mechanismsTransmission of SARS-CoV-2Determining the route of transmission of the SARS-CoV-2 was very crucial to stop the pandemic. The common transmission routes of SARS-CoV-2 include person to-person, direct exposure with cough, sneeze, and droplet inhalation within a range of approximately 1.8 m and transmission through contact with oral, nasal, and eye mucous membrane.62 However, small particles with a larger viral load can be transferred to a distance up to 10 m from the source of emission in an indoor environment.62 Environmental factors including temperature, humidity, and ultraviolet radiation, such as sunlight, are also thought to impact the viability of the virus and its infectiousness over time.63
As epidemiological studies suggested a positive association between air pollution including PM pollution and SARS-CoV-2 infection, it was thought that PM may also act as a carrier for the virus in the outdoor environment. Indeed, previous studies found that influenza virus could be transported by ambient particles.63 Setti and colleagues collected ambient PMs in Bergamo, Italy, and analysed for the presence of SARS-CoV-2 RNA. They found that the particles can contain SARS-CoV-2 RNA in most of collected samples.15 In Turkey, Kayalar and co-workers detected SARS-CoV-2 RNA in approximately 10% of PM samples of various sizes collected in 10 different cities, especially in places where virus spread was common, such as hospital gardens and city centres.16 However, whether these virus particles are infectious or not remains to be determined. Adsorption of the SARS-CoV-2 on airborne dust and PM may be the contributors to the long-range transport of the virus.64
Cellular entry of SARS-CoV-2 and interaction with air pollutantsReceptor recognition is the first step of viral infection, and it has been reported that angiotensin-converting enzyme II (ACE2) is the main cell receptor binding SARS CoV-2.65,66 This enzyme is involved in the renin-angiotensin system (RAS) regulating blood pressure and inflammation. RAS system has 2 pathways oppositely running: the first one is Angiotensin-converting enzyme (ACE)/Angiotensin II peptide (Ang II)/Angiotensin II type 1 receptor (AT1R), which induces pro-inflammatory cytokine (IL-6, TNF-α) release; the second pathway is ACE2 -> Angiotensin 1-7 peptide (Ang1-7) -> Mas receptor, which has anti-inflammatory properties. In the absence of SARS-CoV-2, ACE2 cleaves AngII into Ang1-7 and inhibits the AT1R. Occupation of ACE2 by SARS-CoV-2 binding causes downregulation of receptor and is deemed to shift the pathway in favour of the Ang II axis which leads to an inflammatory response.65 ACE2 receptor is largely expressed in vascular endothelium, respiratory epithelium, alveolar monocytes, and macrophages.66-68 Alveolar type 2 (AT2) cells show the highest ACE2 expression in the lung.69 Moreover, viral entry requires cleavage of spike protein after receptor binding by cellular proteases to allow the fusion of cellular and viral membranes. Transmembrane protease, serine 2 (TMPRSS2) is the major protease utilised by SARS-CoV-2.66-70
Studies demonstrated upregulation of ACE2 and TMPRSS2 proteins because of PM exposure possibly inducing the entry of SARS-CoV-2 into the host cell. Sagawa and co-workers treated mice with PM for 24 hours and showed elevated ACE2 and TMPRSS2 expression in the alveolar regions, especially in AT2 cells.18 A recent study conducted with mice exposed to PM2.5 revealed a 40% increase in ACE2 protein expression in mice lungs.71 According to the “double hit hypothesis” by Frontera et al.,17 chronic exposure to PM2.5, in places such as Northern Italy and the Hubei province of China, caused upregulation of ACE2, as an initial protective mechanism to induce anti-inflammatory pathways. However, ACE2 expression also facilitated viral penetration into the cell that caused viral load increases in the cell. This, in turn, caused depletion of the ACE-2, leading to acute lung injury. The authors suggested that a high level of NO2 in the ambient air caused a second hit, resulting in a severe form of COVID-19.
Studies suggest that air pollutants induce the susceptibility of human beings to viral infections by altering the host immune defence system.72 Mouse studies found that exposure to diesel exhaust induced respiratory syncytial virus (RSV) gene expression in mice infected with the virus.73 It is known that short-term exposure to pollutants causes a pro-inflammatory response together with elevated reactive oxygen species (ROS) production inducing apoptosis, while long-term exposure can be a reason for immune dysregulation leading to different diseases. It has been confirmed that the innate immune capacity of alveolar macrophages is reduced in extremely polluted city populations because of the relative amount of phagocytosed PM.74 For instance, a study containing co-culture of RSV-infected bronchial epithelial cells and PM10 exposed macrophages showed that viral antigen uptake in macrophages in response to the virus dropped to half compared to the control macrophages.75 T-cell-mediated adaptive immune response is an essential part of the continuance of the clearing and suppression of viral infections in the long term. However, viral infections (including COVID-19) may lead to decreased production of T cells, natural killer cells, and interferon (IFN)-γ secretion by CD4+ T lymphocytes.76,77 Regarding air pollution, PM was found to have a reducing effect on IFN-β, a key antiviral cytokine, by affecting its promoter activity, and subsequent transcription. Additionally, diesel exhaust particles (DEP), which are present in ambient atmosphere as ultrafine particles, attenuated IL-2 and IFN-γ production by peripheral blood T lymphocytes causing defective immune status.78 These processes would influence the pathogenicity of SARS-CoV-2. Mice experiments showed that PM can cause alveolar collapse due to bio-mechanical changes occurring in surfactant function. Surfactant deficiency causes acute respiratory distress syndrome (ARDS). Hence, people living in places with higher PM concentrations could have more severe COVID-19 infection due to dysfunctional surfactants, together with ARDS.74
Airway epithelium forms the first line of defence against inhaled insults, including infectious agents. It has been shown that pollutants disrupt the epithelial barrier integrity through occludin reduction in plasma membranes and dissociation of Zonula occludens (ZO)-1, which may allow higher levels of SARS-CoV-2 entry into the host cell. In fact, SARS-CoV-2 itself may cause decreased epithelial barrier integrity, together with impaired tight junctions. Dysfunctional barrier can increase the risk for COVID-19 morbidity. Studies of air pollutants reported that O3 and NO2 increased permeability of human primary bronchial epithelial cell cultures, and that cells from asthmatic subjects were more susceptible to this process. Disrupted muco-ciliary clearance is also one of the significant reasons for an inadequate immune defence system.77 Earlier studies by Bayram et al. demonstrated that DEP could decrease ciliary beat frequency (CBF) and induce release of inflammatory mediators, while modulating cell cycle progression and apoptosis of human airway epithelial cells in several studies.79-81 Recent animal model and in vitro studies showed that the ciliary structure is shortened and deteriorated due to SARS-CoV-2 infection, thus causing changes in ciliary function leading to impaired muco-ciliary clearance in airways.82
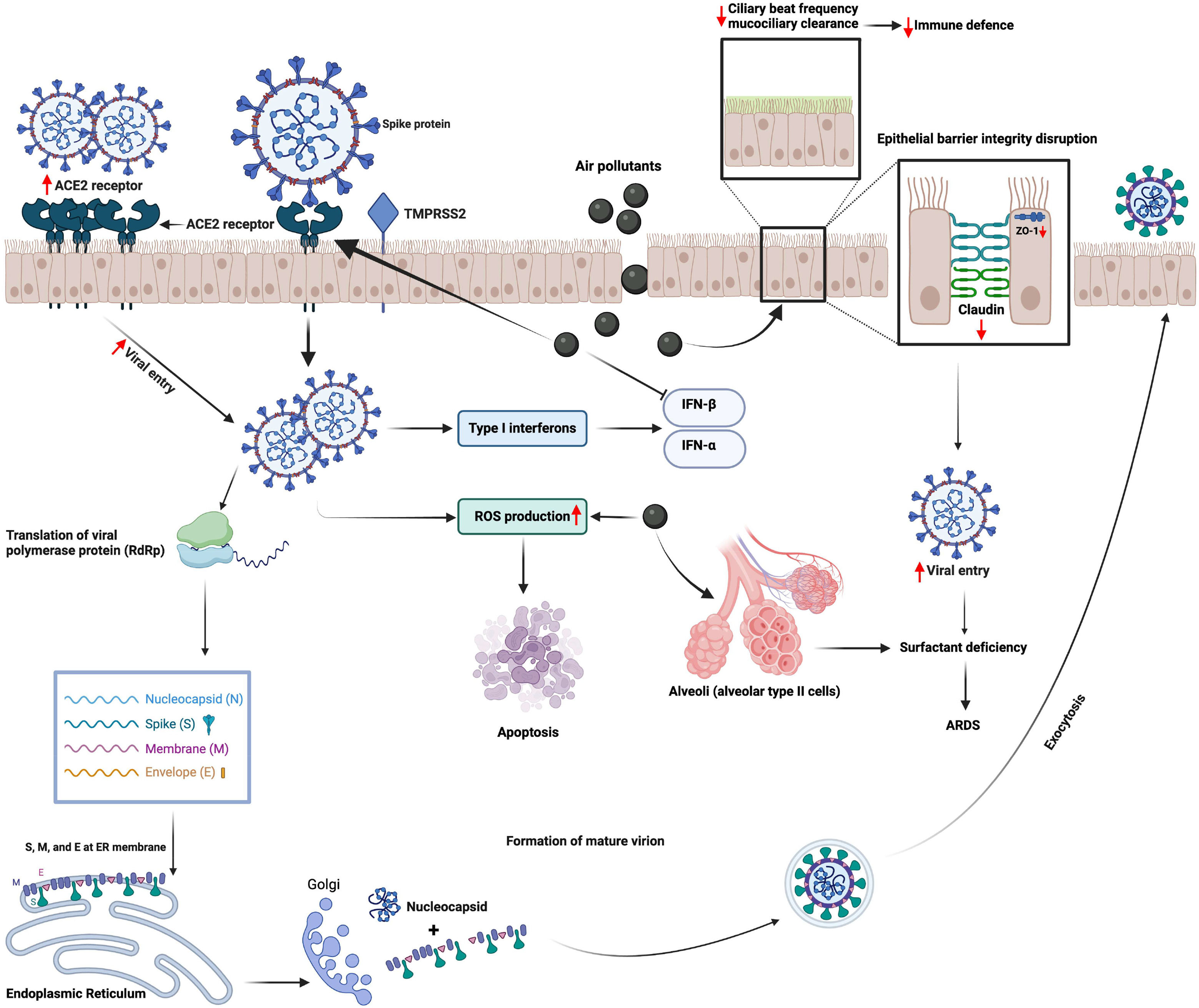
Another aspect focuses on microbiome diversity and how it is altered in SARS-CoV-2-infected individuals. Microbiota of the respiratory tract is an essential part of immunity development since birth; it is known that environmental factors like pollutants have an alteration effect on the diversity of these organisms.77 RNA sequencing research, conducted in the US with COVID-19 patients and a control group, demonstrated differences in diversity and abundance of some bacterial species in the upper respiratory tract microbiome, in which viral and bacterial interaction can influence the viral load, host immune response, and acute severity.83Fig. 1 demonstrates mechanisms underlying the interaction between air pollution and SARS-CoV-2 infection at cellular level.
Mechanisms underlying the interaction between air pollution and SARS-CoV-2 infection at cellular level. Air pollutants disrupt the epithelial barrier integrity together with reducing ciliary beat frequency that impairs mucociliary clearance and facilitates the viral entry. Air pollutants also augment the entry of SARS-CoV-2 by inducing the expression of the angiotensin converting enzyme (ACE)2 receptor. Furthermore, they induce the production of reactive oxygen species (ROS) in the host cell, which could lead to cellular injury and apoptosis. Viral proliferation together with increased levels of ROS triggers cellular damage that could lead to severe clinical conditions such as acute respiratory distress syndrome (ARDS). IFN, interferon; TMPRSS2, Transmembrane protease, serine 2. (The figure is original and was created using BioRender.com).
In summary, studies investigating mechanisms underlying the interaction between air pollution and SARS-CoV-2 infection have suggested that air pollutants can induce the expression of several proteins, which modulate cellular entry of the virus. Furthermore, air pollutants-induced mucociliary dysfunction, epithelial barrier disruption, oxidative stress and inflammatory changes within the cell may also enhance viral proliferation and cellular injury by SARS-CoV-2. However, there is a lack of evidence demonstrating the direct interaction of air pollutants with SARS-CoV-2, and further in vitro and in vivo mechanistic studies are clearly needed.
ConclusionIn conclusion, epidemiological studies demonstrate that air pollutants can play an important role in COVID-19-associated mortality and morbidity, including hospitalisation, and admittance to ICU. Air pollutants can facilitate the entry of SARS-CoV-2 into the host cell, by inducing expression of cellular proteins such as ACE2 and TMPRSS2. Air pollutants can also impair mucociliary function, epithelial integrity, and airway defence mechanisms. Altered microbiome and dysregulated immune system by air pollutants may also be other plausible mechanisms. Finally, PM pollutants may act as carriers for long-range transport of SARS-CoV-2.