Adjuvant platinum-based chemotherapy for completely resected non-small cell lung cancer is associated with modest improvement in survival; nevertheless, no validated biomarker exists for predicting the benefit or harm of adjuvant platinum-based chemotherapy.
Materials and methodsWe simultaneously measured 27 cytokines in operative tumor specimens from a discovery cohort (n = 97) by multiplex immunoassay; half of the patients received adjuvant platinum-based chemotherapy, and the other half were observed. We tested possible prognostic and predictive factors in multivariate Cox models for overall survival (OS) and relapse-free survival (RFS), and a tree-based method was applied to detect predictive factors with respect to RFS. The results were validated in an independent validation cohort (n = 93).
ResultsFifty-two of 97 (54 %) patients in the discovery cohort and 50 of 93 (54 %) in the validation cohort received adjuvant chemotherapy; forty-four (85 %) patients in the discovery cohort and 37 (74 %) in the validation cohort received four cycles as planned. In patients with low IL-1β-expressing tumors, RFS and OS were worse after adjuvant chemotherapy than after observation. The limited effect of adjuvant chemotherapy for patients with low IL-1β-expressing tumors was confirmed in the validation cohort. Additionally, RFS and OS were prolonged by adjuvant chemotherapy only in patients with high IL-1β-expressing tumors in the validation cohort.
ConclusionsThis study identified and validated low tumor IL-1β expression as a potential biomarker of a limited response to adjuvant platinum-based chemotherapy after complete resection of pulmonary adenocarcinoma. This finding has the potential to inform adjuvant treatment decisions.
Following complete surgical resection of non-small cell lung cancer (NSCLC), 5-year distant relapse rates range from 15 % for stage IA to 60 % for stage IIIA,1 suggesting the presence of micrometastases at early stages. Adjuvant platinum-based chemotherapy (ACT) remains an essential component of adjuvant treatment for patients with postoperative stages II and IIIA disease. A pooled analysis by the lung adjuvant cisplatin evaluation (LACE) collaborative group demonstrated a small improvement of 5.4 % in OS at 5 years in ACT patients compared to patients without ACT.2 The TNM classification system is the only clinically established predictor of benefit from ACT.3-5 Evidence suggests that the cytokine signature in the tumor microenvironment is a determinant of the response to chemotherapy6,7 and that the modulation of intratumoral cytokines may be associated with a decreased response to chemotherapy.8 The aim of our study was to identify and externally validate a cytokine signature for predicting a survival benefit from ACT.
Material and methodsParticipantsA data analysis plan was developed prior to the start of the study. The inclusion criteria were complete resection of lung adenocarcinoma, postoperative stages II and IIIA (7th edition), no other previous cancer, and surgery between 2004 and 2013 at Thoraxklinik Heidelberg, Germany. The plan was to include 100 patients randomly in the study. Three of these 100 patients were subsequently excluded from the analysis due to technical difficulties in performing the BioPlex analyses. To this end, 97 patients remained in the discovery cohort. An independent group of 93 patients who underwent surgery for lung adenocarcinoma at Thoraxklinik Heidelberg between 2010 and 2013 was randomly selected using the same criteria. All patients provided written informed consent for the research project, which was approved by the Ethics Committee of the University of Heidelberg (approval numbers S-515/2013 and S-270/2001).
Surgery and postoperative follow-upSystematic mediastinal and hilar lymph node dissection was performed in all patients. Postoperative follow-up consisted of a chest computed tomography scan every six months.
Adjuvant platinum-based doublet chemotherapyThe decision for or against ACT was made in all patients after the patient was seen by a medical oncologist and discussed by a multidisciplinary tumor board. Patients with pathological stage II or IIIA disease received adjuvant chemotherapy within 4 to 8 weeks after surgery. These patients received four cycles of cisplatin (75 mg/m2) with primarily vinorelbine or paclitaxel as a combination of chemotherapeutic agents.9 Alternatively, cisplatin was replaced by carboplatin in patients with comorbidities. Overall, despite a formal indication for ACT, 88 of 190 patients (46 %) did not receive ACT. The reasons for not receiving chemotherapy were similar in both cohorts. In 40 of the 88 patients (45 %), ACT was refused by the patient after personal consultation with a medical oncologist; in 18 patients (20 %), there were significant comorbidities, mainly renal insufficiency; in 13 patients (15 %), the adjuvant interval was missed due to delayed postoperative recovery; and in 6 patients (7 %), age older than 80 years was the deciding factor. In 2 patients (3 %) with pT2pN0 stage IB disease according to the TNM 6th edition which was reclassified to stage IIA according to the TNM 7th edition, 1 patient with node-negative pT3 tumor, and 1 patient with lepidic adenocarcinoma, less severe disease was assumed, and ACT was omitted. The reason for refusal of chemotherapy was missing for 7 patients.
Survival definitionsWe defined OS as the time from surgery to death (event) or the patient's last follow-up (censoring) in months, regardless of the cause of death. Relapse-free survival (RFS) was defined as the time from surgery to locoregional or distant tumor relapse, death (event) or the last follow-up (censoring) in months.
Luminex immunoassayCryosections of frozen tumor tissue samples stored at −80 °C and obtained from the prospectively maintained Lung Biobank Heidelberg were stained with hematoxylin and eosin. Only tumor samples with a content of more than 50 % viable tumor cells were considered for BioPlex analyses. The concentrations of cytokines in the tumors were measured using multiplex technology (Bio-Rad Laboratories, Inc., Hercules, California, USA) as described previously.10 Samples from patients in the validation cohort were analyzed in the same manner as those in the discovery cohort.
Statistical analysesThe prognostic effects of the 27 cytokines were investigated via multivariate Cox models for OS and RFS. Separate models were fitted for each cytokine, each adjusted for age, smoking status, tumor size (pT3 vs.11 The same set of models was fitted to evaluate predictive factors by adding an interaction term for the respective cytokine and treatment. P values were again adjusted for multiple testing. For cytokine clusters, principal component scores were tested via multivariate Cox models for OS and RFS, adjusted for age, smoking status and tumor size.
A tree-based approach was used in addition to identifying predictive factors for ACT.12 The endpoint for this analysis was RFS at 36 months after surgery. An ensemble of 1000 predMOB trees was generated, with each tree on a subsample of the training cohort containing 57 patients using the same factors as before for adjustment. Candidate cytokines were derived based on permutation importance and validated in an external cohort. Predicted survival curves were drawn (Supplementary Fig. 1). Median follow-up times were calculated using the reverse Kaplan‒Meier estimate.13 Descriptive statistics included restricted mean survival time (RMST)14,15 with a follow-up of 5 years.
ResultsDiscovery cohortA total of 97 patients who met the inclusion criteria were included in the BioPlex analyses. Fifty-two (53.6 %) patients received ACT, and 45 (46.4 %) did not receive any adjuvant treatment. Forty-four of 52 (84.6 %) patients received the full dose of the planned 4 cycles. The most frequently administered adjuvant combinations were cisplatin and vinorelbine in 29 (55.7 %) patients and carboplatin and vinorelbine in 22 (42.3 %) patients. Of the 29 patients who started cisplatin therapy, 6 (20.7 %) patients switched from cisplatin to carboplatin after one or two cycles due to adverse events. ACT was discontinued prematurely in 7 patients after 1 or 2 cycles; 3 patients experienced adverse events from cisplatin, 2 patients experienced adverse events from carboplatin, and 2 patients experienced tumor progression.
Validation cohortIn the validation cohort, 93 patients met the inclusion criteria and were available for BioPlex analyses. ACT was administered to 50 (53.8 %) patients, 37 (74 %) of whom received the full dose of 4 cycles, and 7 of these 37 patients who received cisplatin therapy were switched to carboplatin after one or two cycles because of adverse events. Forty-three of the 93 (46.2 %) patients were observed. The most frequently administered adjuvant agents were cisplatin and vinorelbine in 30 (60 %) patients and carboplatin and vinorelbine in 19 (38 %) patients. ACT was discontinued prematurely in 13 patients, including 6 patients due to adverse events resulting from cisplatin, 1 patient due to adverse events resulting from carboplatin, 5 patients due to adverse events despite switching therapy from cisplatin to carboplatin, and 1 patient due to tumor progression (Table 1).
Comparison of demographic and clinical characteristics.
AJCC, American Joint Committee on Cancer; UICC, Union for International Cancer Control.
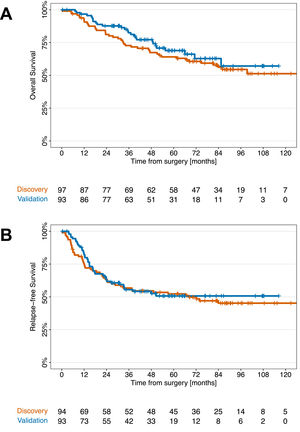
The median follow-up times in the discovery and validation cohorts were 89.0 and 59.3 months, respectively. Fifty-four (55.7 %) patients after ACT and 43 (43.3 %) patients observed developed tumor relapse in the discovery cohort. In the validation cohort, tumor relapse was observed in 47 (50.5 %) patients after ACT and 46 (49.5 %) patients after observation. Distant metastases were the most common type of tumor relapse and occurred in 30 (33 %) patients in the discovery cohort and in 30 (32.2 %) patients in the validation cohort. Metachronous second cancers were detected in 9 patients in the discovery cohort and in 7 patients in the validation cohort. OS and RFS were comparable between the discovery and validation cohorts (Fig. 1). The two cohorts differed in some characteristics. The validation cohort included fewer patients with lymph node metastases (pN1), fewer patients who underwent pneumonectomy, and a lower proportion of patients who died in the validation cohort, which had a shorter follow-up period than did the discovery cohort. In our multivariable analyses, we adjusted for lymph node status to account for this potential confounding factor. Pneumonectomy was associated with a greater hazard for recurrence and/or death (hazard ratio OS: 2.47 and hazard ratio RFS: 3.51), but the addition of pneumonectomy as a factor in the multivariable models for OS and RFS did not change the effect of ACT. We constructed multivariable models with clinical factors, including ACT and excluding IL-1β. As shown in Supplementary Table 1, ACT had no significant effect on OS or RFS compared to observation in either the discovery or validation cohorts (Supplementary Fig. 2).
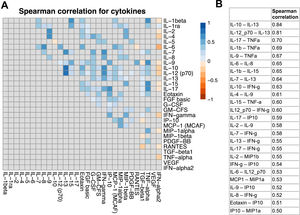
Effects of cytokines on survival in the discovery cohortThe expression of all 27 cytokines in tumors was comparable between the cohorts (Fig. 2). Pairwise correlations between cytokine levels were mostly weak or not present (Fig. 3a). Only IL-13 showed a strong positive correlation with IL-10 and IL-12 (Fig. 3b). Accordingly, principal component analysis revealed no evidence for a grouping of cytokines (Supplementary Fig. 3).
In the discovery cohort, none of the cytokines were found to have a prognostic or predictive effect. No prognostic or predictive effects were found for common cytokine clusters (Supplementary Table 1).
In contrast to the results of the primary multivariate regression models, the results of an alternative, data-driven approach in which model-based random forests were used for the identification of predictive factors suggested the presence of three promising cytokines: basic FGF, IL-17 and IL-1β. These three candidates were subsequently analyzed in an independent cohort of patients for validation.
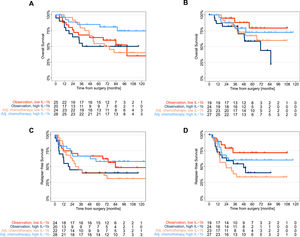
ValidationOf the three candidates, only tumor IL-1β had a significant treatment effect according to univariate and multivariate analyses adjusted for age, tumor size, lymph node status, and smoking status (Supplementary Table 2). Thus, in patients with low tumor IL-1β levels, ACT was not beneficial, while in patients with high tumor IL-1β expression, ACT significantly improved survival (Fig. 4). Because median survival was often not achieved, we additionally calculated the restricted mean survival time (RMST)14 up to 5 years to illustrate the treatment effect of ACT (Table 2).
Survival stratified by IL-1β expression and postoperative treatment.
Kaplan‒Meier curves for the discovery (a, c) and validation (b, d) cohorts comparing overall survival and relapse-free survival between patients who received adjuvant platinum-based chemotherapy and those who received observation stratified by median tumor IL-1β expression as determined in the discovery cohort (high: IL-1β > 14.26 pg/mg; low: IL-1β ≤ 14.26 pg/mg); ACT, adjuvant platinum-based chemotherapy.
Descriptive survival statistics for the discovery and validation cohorts.
CI, confidence interval; NR, not reached; OS, overall survival; RFS, relapse-free survival; RMST, restricted mean survival time; yr, year.
In the discovery cohort, the RMST for RFS in patients with low tumor IL-1β expression was 36 months following ACT, whereas the RMST for RFS was 44 months following observation alone. The limited effect of ACT in patients with low tumor IL-1β levels was confirmed in the validation cohort, in which the RMST for RFS within 5 years was 32 months following ACT and 48 months after observation. The predictive value of low tumor IL-1β expression for OS was not as striking as that for RFS. The five-year OS rates at low tumor IL-1β expression were comparable between the ACT cohort and the observation cohort in the discovery cohort and in the validation cohort. In summary, in patients with low tumor IL-1β expression, the RMST for RFS was shorter in the discovery cohort than in the validation cohort (8 months and 16 months, respectively). In contrast, the RMST for RFS in patients with high tumor IL-1β expression after ACT was 13 months longer in the discovery cohort and 5 months longer in the validation cohort than in the observation cohort (Table 2).
DiscussionWith the perioperative use of targeted therapies and immunotherapies as monotherapy or in combination with chemotherapy in resectable NSCLC, the use of isolated chemotherapy in the adjuvant setting will play a more limited role. Therefore, it is important to identify biomarkers that can predict who will benefit most from chemotherapy, allowing for increasingly personalized therapy. To date, the indication for ACT in completely resected lung adenocarcinoma has been based on postoperative tumor stage, with minimal predictive value regarding the survival benefit of ACT. Contrary to what would be expected from ACT, we found that in patients with tumors expressing low levels of IL-1β, standard platinum-based ACT not only conferred no survival benefit but also conferred a potential detrimental effect on RFS. During the 5-year follow-up, in patients with low tumor IL-1β expression, the RFS time after ACT was 8 months shorter in the discovery cohort and 16 months shorter in the validation cohort than in the postoperative observation cohort. Confusingly, by visually interpreting the shape of the Kaplan‒Meier curves (Supplementary Fig. 2), ACT appeared to be associated with worse RFS than was observation in the validation cohort. We explain this difference by the fact that important prognostic factors were not included in the calculation of the Kaplan‒Meier curves. In addition, the small number of patients at risk, the overlapping 95 % confidence intervals and the small proportion of stage III patients contributed to the appearance of the curves. According to the LACE meta-analysis,2 which included more patients with squamous cell carcinoma than with lung adenocarcinoma, the hazard ratio for recurrence or death with ACT versus observation showed that ACT had the greatest effect on stage III disease. In this study, only 5 of the 97 patients (5.2 %) in the discovery cohort and none of the patients in the validation cohort had stage III disease.
The ADAURA trial, which showed a RFS16 and OS17 benefit of adjuvant osimertinib in patients with EGFR mutation-positive lung adenocarcinoma compared with placebo, and the immunotherapy trial by Felip et al.,18 in patients treated with adjuvant atezolizumab compared with best supportive care, led us to question the clinical value of IL-1β as a biomarker for conventional ACT. However, in adjuvant treatment, targeted therapies and immunotherapies do not completely replace ACT. Therefore, given the high proportion of patients in whom ACT is still indicated postoperatively, biomarkers for assessing ACT are clinically meaningful.19 The hazard ratio for OS after ACT was greater than 1.0 for patients with a tumor IL-1β concentration less than ∼12 pg/mg (Supplementary Fig. 4a) and for RFS after ACT for patients with a tumor IL-1β concentration less than ∼21 pg/mg (Supplementary Fig. 4b). Sixty-two of 97 (65 %) patients in the discovery cohort and 55 of 93 (59 %) patients in the validation cohort had tumor IL-1β levels less than ∼21 pg/mg. The importance of a predictive biomarker for ACT to avoid overtreatment with potential toxicities was demonstrated by the LACE meta-analysis.2 Although pooled analysis revealed a 5.4 % and 5-year OS improvement in patients receiving ACT compared with observation, subgroup analysis of patients with stage IA disease revealed that ACT had a limited effect (HR, 1.40), which is why ACT is not recommended for patients with stage IA disease. Since then, numerous studies have investigated potential biomarkers for predicting the benefit of ACT in completely resected NSCLC20-24; however, to date, none of these biomarkers or gene signatures have been established to play a role in predicting the benefit of ACT.25
Cisplatin and vinorelbine have been the standard combination for ACT since 20049,26 and were administered to 56 % and 60 %, respectively, of patients in the discovery and validation cohorts. Carboplatin and cisplatin offer comparable survival benefits, although no randomized trial has yet directly compared carboplatin with cisplatin in the adjuvant setting.27 Among patients who received a full 4 cycles of ACT, 18 % (8 of 44 patients) in the discovery cohort and 19 % (7 of 37 patients) in the validation cohort were switched from cisplatin to carboplatin.
IL-1β, one of 11 members of the IL-1 family, is activated by a specific inflammasome and has been shown to be highly expressed in lung cancer. While it promotes inflammation-induced carcinogenesis, it can also recruit antineoplastic cells and thereby block the growth of metastases. IL-1β is associated with numerous pathological conditions of the lung, such as COPD, emphysema and COVID-19.28,29 We found no associations between tumor IL-1β expression and clinical parameters in either cohort (Supplementary Fig. 5).
The randomized, phase III CANTOS trial30 of 10,061 patients with atherosclerosis revealed a surprising dose-dependent 67 % reduction in lung cancer incidence and a 77 % reduction in lung cancer-related mortality with canakinumab, a human monoclonal antibody with high affinity and specificity for IL-1β,31 compared to placebo. The results of the CANTOS trial highlighted the role of IL-1β in the pathogenesis of lung cancer. Although the mechanistic basis of IL-1β blockade is still unclear, the first data from randomized controlled trials on the perioperative use of IL-1β blockade in resectable NSCLC patients are now available. The phase II neoadjuvant CANOPY-N trial, which evaluated canakinumab as monotherapy or in combination with pembrolizumab in 88 patients with resectable NSCLC prior to planned surgery, did not meet its primary endpoint of major pathologic response.32 Similarly, the addition of canakinumab to surgery and cisplatin-based ACT in patients with completely resected (R0) stage II-III NSCLC did not meet the primary endpoint of disease-free survival in the multicenter, double-blind, placebo-controlled, randomized phase III CANOPY-A trial, which included a total of 1382 patients.33 In both studies, IL-1β blockade had an impact on markers of inflammation, although it did not demonstrate clinical activity in resectable NSCLC. Several factors, such as the different patient populations, may explain the disparate results of CANTOS and CANOPY-A. The upcoming molecular analyses in CANOPY-A may help to better classify the unexpected lack of benefit of IL-1β blockade in the adjuvant setting.
The association between IL-1β expression and survival after ACT is not apparent. One explanation for the limited effect of ACT in patients with low tumor IL-1β expression could be immunocompetence, which is crucial for the efficacy of chemotherapy34; however, surgical factors such as activation of the sympathetic nervous system, removal of lymph nodes and anesthetics can impair immune function.35 It is believed that the immunosuppressive state after surgery can last from a few days to 6 months.36 In this study, patients who did not receive ACT and had low tumor IL-1β expression had the best RFS, regardless of whether they received ACT (Fig. 4b and d). Especially in the immunologically vulnerable postoperative phase, the immunosuppressive effect of ACT had a negative impact on otherwise favorable outcomes. Similar results for ACT were reported in patients with p53-negative tumors who were randomized to receive either four cycles of adjuvant cisplatin plus vinorelbine or observation alone in a phase III study of patients with completely resected stage IB and II NSCLC.22 Another explanation for the lack of survival benefit of ACT in patients with low tumor IL-1β expression may be the tumor immune microenvironment, which can influence the response to chemotherapy.6 In a mouse model, the induction of antitumor immune responses and tumor suppression by the anthracycline analog mitoxantrone was dependent on the expression of the tumor suppressor gene phosphatase and tensin homolog deleted on chromosome 10 (PTEN) on myeloid cells. PTEN interacts with the nucleotide oligomerization domain (NOD)-like receptor pyrin domain containing-3 (NLRP3), which is the most frequently activated inflammasome by agents such as cisplatin and is required for inflammasome assembly, T-cell infiltration, and activation in the tumor microenvironment. Supplementation with IL-1β restored chemotherapy sensitivity in PTEN-deficient mice and established a link between IL-1β and chemotherapy-induced antitumor immunity. The authors concluded that manipulation of the NLRP3-IL-1β axis may be able to overcome chemotherapy resistance in PTEN-deficient tumors.37 PTEN expression was not measured in the cohorts in this study. However, PTEN loss in NSCLC has been reported in up to 45 % of tumors in various studies.38,39 It is therefore possible that the expression of IL-1β is necessary to increase chemosensitivity to cisplatin, which may not have been present in a large proportion of patients in our study due to a potential lack of PTEN expression.
This study has several limitations. First, because of its retrospective nature, the study was not randomized. Second, the samples were stored over a long period between 2004 and 2013, which may limit the reliability and comparability of the results. Third, cytokine levels were determined in frozen tumor samples and may differ from cytokine levels in fresh tissue due to the dynamic state of cytokines.
The only biomarker for the effect of ACT that can be used for benchmarking is the TNM classification. However, the published data for the TNM classification are mostly based on squamous cell carcinoma, include other tumor stages and involve the use of different chemotherapy regimens.2 Our results are based on nonrandomized data, but the findings are strengthened by adjustment for standard prognostic parameters,40 the requirement for a high level of significance, and the use of an external validation cohort. In summary, our results indicate that patients with low tumor IL-1β expression do not benefit from ACT in terms of survival. In patients with tumors with high IL-1β expression, additional therapy seems reasonable, as these patients are likely to receive a survival benefit from ACT.
ConclusionsIL-1β could be an independent tumor biomarker for the effect of ACT on survival. IL-1β could be used to identify patients who are unlikely to benefit from ACT after complete resection of lung adenocarcinoma.