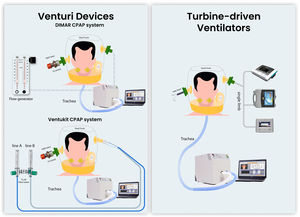
Traditionally, Venturi-based flow generators have been preferred over mechanical ventilators to provide continuous positive airway pressure (CPAP) through the helmet (h-CPAP). Recently, modern turbine-driven ventilators (TDVs) showed to be safe and effective in delivering h-CPAP. We aimed to compare the pressure stability during h-CPAP delivered by Venturi devices and TDVs and assess the impact of High Efficiency Particulate Air (HEPA) filters on their performance.
MethodsWe performed a bench study using an artificial lung simulator set in a restrictive respiratory condition, simulating two different levels of patient effort (high and low) with and without the interposition of the HEPA filter. We calculated the average of minimal (Pmin), maximal (Pmax) and mean (Pmean) airway pressure and the time product measured on the airway pressure curve (PTPinsp). We defined the pressure swing (Pswing) as Pmax - Pmin and pressure drop (Pdrop) as End Expiratory Pressure - Pmin.
ResultsPswing across CPAP levels varied widely among all the tested devices. During “low effort”, no difference in Pswing and Pdrop was found between Venturi devices and TDVs; during high effort, Pswing (p<0.001) and Pdrop (p<0.001) were significantly higher in TDVs compared to Venturi devices, but the PTPinsp was lower (1.50 SD 0.54 vs 1.67 SD 0.55, p<0.001). HEPA filter addition almost doubled Pswing and PTPinsp (p<0.001) but left unaltered the differences among Venturi and TDVs systems in favor of the latter (p<0.001).
ConclusionsTDVs performed better than Venturi systems in delivering a stable positive pressure level during h-CPAP in a bench setting.
Noninvasive respiratory support strategies have been widely used for managing Acute Hypoxemic Respiratory Failure (AHRF), especially during the COVID-19 pandemic.1-5 Particularly, the application of continuous positive airway pressure (CPAP) through a helmet (h-CPAP) is more effective in lowering the respiratory rate, improving gas exchange and reducing the length of stay compared to face-mask.6-8 with the advantage of minimizing pressure ulcers,9 and mitigating leaks. Thus, h-CPAP equipped with High Efficiency Particulate Air (HEPA) filters has become very popular in treating patients with COVID-19-related AHRF10-13 since it minimizes the risk of environmental aerosolization14,15 and is feasible, even outside the intensive care unit (ICU).10,16
Traditionally, Venturi-based flow generators have been preferred over ICU ventilators17-19 to provide h-CPAP since they generate continuous gas, as required, to keep the positive pressure constant throughout the respiratory cycle and avoid carbon dioxide (CO2) rebreathing.20 Moreover, the first generations of mechanical ventilators imposed high levels of inspiratory effort during CPAP triggering.21-23 Conversely, h-CPAP was considered not feasible using high pressure or turbine-driven ventilators (TDVs) equipped with a double-limb circuit due to the lack of sufficient flow to flush CO218, so that a warning was issued about the use of closed-circuit ventilators with helmet.17 Recently, the introduction of high-performance modern TDVs able to generate up to 240 L/m of inspiratory flow24 in single-limb circuit configuration has emerged as a feasible strategy to support the helmet.25,26
We aimed to compare the pressure stability during h-CPAP delivered by Venturi devices and TDVs, at different levels of patient effort. The secondary aim was to assess the impact of HEPA filters on the performance of h-CPAP with tested devices.
Materials and methodsThe bench study was performed using an artificial lung (LS-ASL-5000, Ingmar Medical, USA) set to mimic a restrictive respiratory impairment (resistance 7.5 mH2O/L/s, compliance 30 ml/cmH2O, semisinusoidal waveform with rise time of 25%, inspiratory hold of 5%, and release time of 25%), simulating two different levels of patient effort: 1) “high effort” (20 cmH2O) with a respiratory rate of 30 breaths/min, and 2) “low effort” (12 cmH2O) with 22 breaths/min, as previously described.26,27 A modified mannequin trunk (Laerdal Medical AS) was connected to the artificial lung simulator. A medium-size helmet (Starmed Ventukit or CaStar, Intersurgical, Italy) was secured over the head and sealed with standard straps under the mannequin armpits. Unintentional leaks were avoided by proper fixing and sealing the helmet to the mannequin's neck.
We compared the performances of h-CPAP delivered by 1) CPAP Venturi system (DIMAR, Italy); 2) Ventukit (Intersurgical, Italy); 3) three different TDVs: Astral 150 (Resmed, USA), Trilogy Evo (Philips, Respironics, USA), Vivo 65, (Breas, Sweden). All measurements were recorded with and without HEPA filters (F1 DAR Covidien with a resistance of 0.8 cmH2O at 30 L/min) placed in the inspiratory and expiratory ports (except for Ventukit, where the inspiratory port is sealed to the Venturi system). Experimental set-up is shown in Fig. 1. The experiments were reproduced with “high” and “low” patient effort settings, as previously defined.
The CPAP level was set at 10 cmH2O for all the experiments with a fraction of inspired oxygen of 40%. The Ventukit was driven by two oxygen lines (line A+B) set at a flow of 10+0 l/m, respectively; the DIMAR Venturi system was set at 9+8 l/m (line A+B) and 11+13 l/m (line A+B) as suggested by the manufacturer in case of HEPA filter interposition. A fixed pre-calibrated 10 cmH2O positive end-expiratory (PEEP) valve (Harol, Italy) was applied to the helmet expiratory port. The turbine-driven ventilators were set in CPAP mode at 10 cmH2O in a single-limb configuration with an intentional leak of 6 mm (approximately 40 l/m of leak flow at this level of PEEP) placed at the helmet expiratory port, as previously described.26 All the devices underwent testing and calibration according to the manufacturer's indications before each measurement, with and without the filters in place as for the experimental setup.
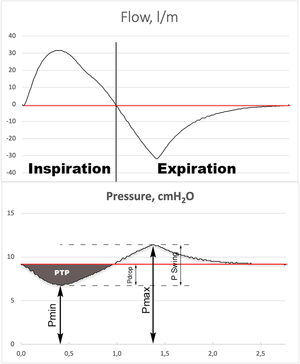
MeasurementsData were collected during the last 2 minutes of a 3-minute recording to achieve data stabilization. Data acquisition was performed at 512 Hz and stored on a personal computer. Offline measurements and curve analyses were performed on a breath-by-breath basis. All measures were performed at body temperature and atmospheric pressure and saturated with water vapor conditions. We calculated the average of minimal (Pmin), maximal (Pmax) and mean (Pmean) airway pressure of the recorded time frame. We defined pressure swing (Pswing) as Pmax - Pmin and pressure drop (Pdrop) as End Expiratory Pressure - Pmin (Fig. 2). The inspiratory pressure time product (PTPinsp) measured on the airway pressure curve was calculated as the area under the PEEP level from the onset to the end of the inspiratory flow. This is an index of the capability of the interface to maintain the airway pressure constant at the PEEP level during the inspiration7. The PTPinsp was calculated by RespiSim System ASL 5000 software 3.6 (Ingmar Medical, USA).
Illustration of graphic measurement of minimum inspiratory pressure (Pmin), maximum expiratory pressure (Pmax) and mean pressure (Pmean), (red line). The PSwing is defined as: Pmax – Pmin. The Pdrop is defined as: Pmean – Pmin. The gray shade area represents the inspiratory pressure time product (PTPinsp).
Descriptive statistics are expressed as mean and standard deviation (SD) for continuous variables. We conducted a sub-group analysis dividing the dataset into two groups according to device type (Venturi devices, TDVs). The Shapiro-Wilk test and histograms were used to analyze variables’ distribution. Normally distributed data were compared with the Student t-test. A P value <0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics for Macintosh, Version 28.0 (IBM Corp Armonk, USA).
ResultsThe analysis of the pressure tracings showed a great variability of the Pswing among all the tested devices (Fig. 3). Overall, pressures and PTPinsp changes between Venturi and TDVs devices in different effort conditions are reported in Table 1. During “low effort”, the Venturi devices and the TDVs did not show any difference in Pswing and Pdrop (Fig. 4), respectively; however, only the TDVs showed a Pmean close to the target CPAP level (p<0.001) associated with a lower PTPinsp (-0.98 SD 0.36 vs -0.71 SD 0.44, p<0.001), and a more positive Pmin (p<0.001).
Performance of each CPAP delivery system, recorded in different experimental settings, without and with High Efficiency Particulate Air (HEPA) filter and in different simulated effort conditions. a) Minimal (Pmin) and maximal (Pmax) airway pressure. The length of the bar represents Pswing (Pmax – Pmin). b) Inspiratory pressure time product (PTPInsp).
Performance of CPAP delivery system grouped by device type (Venturi systems vs TDVs), recorded in different experimental settings, without and with High Efficiency Particulate Air (HEPA) filter and in different simulated effort conditions.
| HEPA Off | HEPA On | |||||||
|---|---|---|---|---|---|---|---|---|
| Venturi | TDVs | p value | Venturi | TDVs | p value | p value* | p value⁎⁎ | |
| Low Effort | ||||||||
| Pmean, cmH2O | 9.37 (0.82) | 10.35 (0.37) | <0.001 | 10.43 (1.32) | 9.96 (0.42) | <0.001 | <0.001 | <0.001 |
| Pmax, cmH2O | 9,63 (0,70) | 10,78 (0,37) | <0.001 | 10.47 (1,23) | 10,27 (0.69) | 0.025 | <0.001 | <0.001 |
| Pmin, cmH2O | 7.53 (0.74) | 8.66 (0.64) | <0.001 | 8.11 (1.64) | 7.71 (0.47) | <0.001 | <0.001 | <0.001 |
| Pdrop, cmH2O | -1.66 (0.27) | -1.67 (0.33) | 0.75 | -2.17 (0.43) | -2.32 (0.15) | 0.065 | <0.001 | <0.001 |
| Pswing, cmH2O | 2.10 (0,09) | 2.12 (0,29) | 0.41 | 2.36 (1.02) | 2.56 (0.33) | 0.01 | 0.003 | <0.001 |
| PTPinsp, cmH2O*s | -0.93 (0.36) | -0.60 (0.44) | <0.001 | -1.36 (0.62) | -1.17 (0.48) | <0.001 | <0.001 | <0.001 |
| Peak Flow, l/m | 33.06 (5.33) | 38.75 (6.96) | <0.001 | 32.43 (8.80) | 35.44 (9.28) | <0.001 | 0.178 | <0.001 |
| Mean Flow, l/m | 20.57 (2.73) | 23.73 (4.44) | <0.001 | 20.68 (5.48) | 22.40 (6.30) | <0.001 | 0.398 | 0.003 |
| High Effort | ||||||||
| Pmean, cmH2O | 8.44 (1.41) | 10.12 (0.56) | <0.001 | 10.92 (0.45) | 9.58 (0.55) | <0.001 | <0.001 | <0.001 |
| Pmax, cmH2O | 9,01 (1,22) | 10,84 (0,91) | <0.001 | 10.93 (0.43) | 10.49 (1.83) | <0.001 | <0.001 | 0.005 |
| Pmin, cmH2O | 5.01 (1.18) | 6.34 (1.10) | <0.001 | 6.62 (0,28) | 5.11 (0.58) | <0.001 | <0.001 | <0.001 |
| Pdrop, cmH2O | -3.50 (0.69) | -4.04 (0.61) | <0.001 | -4.79 (0.29) | -4.81(0.15) | 0,270 | <0.001 | <0.001 |
| Pswing, cmH2O | 4 (0,30) | 4.50 (0,51) | <0.001 | 4.30 (0.19) | 5.37 (1.45) | <0.001 | <0.001 | <0.001 |
| PTPinsp, cmH2O*s | -1.67 (0.55) | -1.50 (0.54) | <0.001 | -2.66 (0.30) | -1.97 (0.61) | <0.001 | <0.001 | <0.001 |
| Peak Flow, l/m | 60.38 (9.18) | 63.43 (7.84) | <0.001 | 54.28 (6.12) | 57.65 (7.58) | <0.001 | <0.001 | <0.001 |
| Mean Flow, l/m | 38.63 (6.34) | 40.21 (5.38) | <0.001 | 34.83 (3.90) | 36.32 (5.39) | <0.001 | <0.001 | <0.001 |
TDVs in the same simulated effort conditions, with HEPA filter Off vs On.
All values are expressed as mean (standard deviation). Pswing= Pmax – Pmin; Pdrop: End Expiratory Pressure – Pmin.
Abbreviations: HEPA, High Efficiency Particulate Air; Pmax, maximal airway pressure; Pmean, mean airway pressure; Pmin, minimal airway pressure; PTPinsp, inspiratory pressure time product; Pswing, pressure swing; Pdrop, Pressure drop; TDVs, turbine-driven ventilators.
Performance of CPAP delivery system grouped by device type (Venturi systems vs TDVs), recorded in different experimental settings, without and with High Efficiency Particulate Air (HEPA) filter and in different simulated effort conditions. Abbreviations: Pswing, pressure swing; Pmin, minimal airway pressure; PTPinsp, inspiratory pressure time product; TDVs, turbine-driven ventilators. *Statistically significant difference.
During the “high effort” condition, all absolute pressures (Pmean, Pmax, Pmin) were below the target CPAP level using Venturi devices. By contrast, when testing TDVs, absolute pressures were distributed across the target CPAP, with Pmean being very close to it (Table 1). Pswing (4 SD 0.30 vs 4.5 SD 0.51, p<0.001) and Pdrop (-3.50 SD 0.39 vs -4.04 SD 0.61, p<0.001) were significantly higher in amplitude with TDVs compared to Venturi devices, but only TDVs were able to lower PTPinsp (1.70 SD 0.55 vs 1.41 SD 0.54, p<0.001).
In a one-by-one analysis of devices without HEPA filters, Pmean and Pmax were consistently lower than the set CPAP level with all Venturi systems apart from DIMAR 11-13 during both effort simulations. Conversely, TDVs were able to guarantee a Pmean around the set pressure (9,94 cm H2O SD 0,57) or higher with EVO. Interestingly, PTPinsp was significantly lower in both effort simulations for all TDVs compared to Venturi systems, with EVO and Astral performing better (Table 1 and Table 2 supplementary material).
The effect of the addition of the HEPA filter is shown in Table 1. High Efficiency Particulate Air filter significantly increased Pswing, Pdrop, and PTPinsp consistently, in both effort conditions (p<0.001). In the “low effort”, the use of HEPA filter in combination with the Venturi devices shifted all absolute pressures (Pmean, Pmax, Pmin) to more positive values (p<0.001), significantly increasing both Pswing and Pdrop (p<0.001); conversely, HEPA filters added to TDVs decreased Pmean, Pmax and Pmin (p<0.001). Nevertheless, Pswing and Pdrop increased, indicating a further HEPA filter-induced detrimental effect also with TDVs. Similar results were obtained in the “high effort” condition (Table 1).
DiscussionThe main results of this bench study, in a model of increased elastic workload, can be resumed as follow:
- •
At low ventilatory demands, both Venturi systems and TDVs are efficient in maintaining a stable pressure.
- •
At high ventilatory demand, TDVs were more effective than Venturi systems in maintaining the target CPAP level while lowering the inspiratory effort.
- •
Addition of HEPA filters improves, in part, CPAP delivery using Venturi devices, but TDVs still perform better.
Theoretically, an ideal CPAP system should maintain airway pressure constant at the set PEEP level during the whole respiratory cycle and this is crucial to prevent alveolar collapse and increase end-expiratory lung volumes. High pressure swings during the inspiratory phase occur when the gas delivered through the helmet fails to meet the patient's inspiratory demands. The higher the patient's inspiratory effort, the higher the pressure swing inside the helmet, particularly if the inlet flow is constant, as it is for Venturi-based systems. Moreover, large pressure swings around the set CPAP level are indirect signs of CO2 rebreathing which also contribute to an increase in patients’ WOB.17,28-30
Previous work showed that ICU ventilators performed worse than Venturi devices in delivering a stable CPAP level, mainly because of the demand-valve technology.19 The latest TDVs are able to generate high flow rates and are equipped with complex algorithms developed to obtain a fast flow modulation,24 instantaneously adapting inspiratory and expiratory flow output to maintain a constant pressure throughout the breath cycle in CPAP mode. H-CPAP delivered using single-limb circuit TDVs, with the intentional leak placed at the helmet expiratory port, using a calibrated hole, showed to be safe and effective.25,26,31
The present study confirms that TDVs are more effective than Venturi devices in maintaining a stable CPAP level when using a helmet in a model of increased elastic inspiratory workload. This effect is even more evident and relevant in the condition of increased breathing effort and when an additional resistive load (HEPA filter) is applied. The finding of a Pmean delivered by TDVs, which is always closer to the target CPAP level than that delivered by Venturi devices, supports this first statement. Accordingly, the added ventilator-induced workload (PTPinsp) was consistently lower using TDVs in any condition tested. These findings in a bench study mimicking acute respiratory failure may be relevant in current clinical scenarios imposed by the COVID-19 pandemic in which AHRF was treated by h-CPAP with various devices with different outcomes.10,31-33
Apparent conflicting results were also found in this study: 1) lower PTPinsp levels during TDVs ventilation despite similar/higher Pswings and more negative Pmin as compared to Venturi systems, with PTPinsp representing the capability of the system to maintain constant the airway pressure at the PEEP level during the inspiration, and 2) a decrease of all recorded pressures during TDVs ventilation with HEPA filters on, whereas they all increased with Venturi devices whatever the inspiratory effort applied. All the positive results about TDVs use in delivery h-CPAP and the above conflicting findings can be explained by the peculiar operating modes of CPAP delivery systems, namely the ability of TDVs to instantly change the delivered flow to correct pressure oscillations according to the set CPAP value, compared with Venturi-based systems which deliver constant flows through a fixed resistance (PEEP valve). In this latter situation, both the increase of ventilatory demands and/or the increase of circuit resistance with HEPA filters reduced all the recorded pressures generated by the Venturi devices well below the target CPAP level, resulting in under-assistance and increased ventilator-induced work of breathing. It is also remarkable that Pmean remained close to the target CPAP in all the conditions in which TDVs were tested, reinforcing the concept that these devices adapt better to sudden changes in ventilatory demands and/or circuit resistance.
Furthermore, it should be noted that, whereas changes in Pmean assess the capability of maintaining a fixed pressure, Pmin and Pmax account for the assessment of transient pressure changes. Accordingly, it becomes clear why Pmin, Pmax, and, hence, Pswing were significantly higher in TDVs compared to Venturi systems. In fact, during h-CPAP delivered by TDVs, all these latter variables tracked pressure changes occurring during inspiratory and expiratory trigger phases respectively, both required for TDVs operativity. Finally, the extra workload imposed by inspiratory-expiratory triggering did not significantly affect either pressure stability around the target CPAP level or the overall ventilator-induced inspiratory workload.
The issue of filter interposition was mostly raised during the COVID-19 pandemic when h-CPAP had been suggested as the first therapeutic choice for the treatment of AHRF related to COVID-19 pneumonia by an expert consensus in the early phase of the outbreak.34 H-CPAP, in fact, decreases unintentional leaks compared to face mask interfaces, potentially minimizing aerosol generation.15,18
A recent bench study showed that the interposition of filters during h-CPAP delivered by Venturi systems significantly reduces the delivered gas flow, which is not sufficient to provide a constant CPAP level during the respiratory cycle.35 Similarly, in an experimental setup, Rezoagli36 showed that filters placed before the PEEP valve of the helmet could increase the resistance to the continuous flow generating additive levels of pressure to the targeted level of PEEP during h-CPAP. Our results confirm that the addition of HEPA filters has detrimental effects on the performances of CPAP devices in terms of pressure stability and added inspiratory and total work of breathing. Indeed, PTPinsp almost doubled with the addition of HEPA filters in any simulated effort condition and for any device tested. Notably, we demonstrated that adding filter resistance has a different impact on the Pmin using different CPAP delivery systems due to their different way of working. In a constant flow system like Venturi devices, the filter represents an additional resistance to the circuit and, according to the Poiseuille equation (Flow=ΔPressure/Resistance), any increase in resistance corresponds with an increase in pressure inside the circuit. In our study, the Venturi systems benefitted from the addition of HEPA filters, the resulting pressures were more positive and, hence, closer to the target h-CPAP. Conversely, fewer positive pressures were recorded using TDVs with the HEPA filter on, because, in TDVs, the constant variable of the Poiseuille equation is the pressure. Notwithstanding this disadvantage, while working with HEPA filters on, TDVs still performed better than Venturi devices in terms of both mean pressure delivered and ventilator-imposed inspiratory workload.
One of the main concerns when delivering CPAP using helmets is the ability to generate enough flow to avoid rebreathing.25 The addition of an intentional leak to the expiratory port of the helmet and the use of last-generation high-performance TDVs prevents rebreathing. As a matter of fact, in any condition tested in the present study, peak and mean inspiratory flows were always significantly higher than the reference device(s) (Venturi systems) (on average +4 l/min and +2 l/min, respectively), irrespective of the presence or absence of HEPA filter (Table 1). These data, coupled with the absolute flows obtained both during the low and high effort conditions (Table 1), preclude the possibility of rebreathing during h-CPAP delivered by the TDVs tested.
These findings offer a better understanding of the functioning of the devices used to deliver h-CPAP, with significant implications for clinical practice, since the bench model may faithfully reflect challenging clinical situations, such as treating a patient with a low compliance and high respiratory drive. Acute hypoxemic respiratory failure, especially when associated with bilateral pulmonary infiltrates due to capillary endothelial injury and diffuse alveolar damage, is characterised by a low lung compliance and, in most cases, by an increased respiratory drive, as simulated in our bench test. Noninvasive strategies such as CPAP may successfully avoid endotracheal intubation.37 The ideal outcome of CPAP application should be improving gas exchanges by promoting alveolar recruitment and reducing the inspiratory effort and the transpulmonary pressure.38 The finding of stable and reliable pressure delivered by TDVs during h-CPAP may theoretically lead to a reduction in transpulmonary pressure variation since the pressure applied to the airway is one of its key determinants, thus stopping the vicious circle of alveolar stretching and transvascular fluid filtration resulting in patient-self-inflicted lung injury (P-SILI). Based on these features and previously published data showing their ability to estimate the delivered tidal volume during h-CPAP,26,31 TDVs are of particular interest, as monitoring tidal volume is crucial to assess the effectiveness of noninvasive respiratory support strategies in assuring adequate alveolar ventilation and avoiding high tidal volume that may contribute to lung injury itself, determining P-SILI, treatment failure, and need for intubation.39,40 The present data fill a research gap by confirming the safety and effectiveness of widely available TDV devices as a potential alternative source of CPAP for everyday clinical use in AHRF and as a practical solution in resource-constrained settings such as low-income countries or pandemics. Moreover, the observed differences among CPAP delivery systems may influence clinicians’ decisions on device selection based on the setting where h-CPAP is delivered (ICU, pre-hospital, emergency department) and on the different clinical situations.
The strengths of the study are the use of a lung model with standard mechanical characteristics that made it possible to closely replicate patients’ work of breathing and systematically assessed pressure stability in various CPAP delivery devices and the influence of filters and patterns of effort. Our choice of lung simulator parameters is within those suggested for simulation studies.41 Furthermore, even if effort model parameters are difficult to evaluate during mechanical ventilation and, hence, h-CPAP,41 the present analysis of two levels of patient effort allowed us to assess h-CPAP devices in conditions of moderate to severe AHRF. Taking into account all of the above, safety and efficacy results of h-CPAP delivered with TDVs described in the present study seem to apply to several clinical conditions that can benefit from this ventilatory support: moderate to severe AHRF caused by COVID-19-related ARDS,42 typical ARDS,41,42 and cardiogenic pulmonary edema.43
This study has several limitations. First, this is a bench study mimicking clinical situations in a strictly controlled environment and only partially reflects the more complex clinical scenarios. Second, only one type of HEPA filter was tested, limiting the generalizability of the results to all the types of filters available on the market. Third, in real-life scenarios, nonintentional leaks at the helmet-neck sealing site can be minimized but not eliminated. Testing of ventilation devices can include testing the role of unintended leaks.43 However, as had been done previously,31 in the present study, we did not test unintentional leaks by sealing the helmet collar to the manikin neck in order to better evaluate the performance of Venturi and TDVs devices without an extremely unstable variable. Fourth, we specifically focused on simulating a restrictive condition model; therefore, no information on devices’ performance in a high resistance model is provided. Fifth, we did not foresee data acquisition with external measurements (i.e. pneumotachograph and/or pressure transducer at the inlet of the helmet). Lastly, even if we simulated a high respiratory rate (30 breaths/min) in the high effort scenario, we cannot exclude the possibility that higher respiratory rates might show further differences in performance between Venturi devices and TDVs. Further studies are needed to understand the clinical impact of these results. Moreover, a bench test comparison of several types of TDVs may better help address clinicians’ choices in real life.
ConclusionsTurbine-driven ventilators performed better than Venturi systems in delivering a stable pressure level during h-CPAP in this bench setup. Overall, the addition of HEPA filters has an expected negative impact on pressure stability, but to a lesser extent, even to be clinically negligible, when the helmet is used with TDVs.
Authors' contributionsAll authors discussed the topic and the idea to perform a bench test; AN and CC performed the bench test; AN, GG managed data curation and conducted the formal analysis; AN, CC and AC interpreted the data; AN and CC wrote the original draft; AC, CC, LA, CG and ACa critically revised the draft; all authors read and approved the final version of the manuscript.
Availability of data and materialAvailable on request without undue reservation.
Ethics approvalNot applicable.
Consent to participateNot applicable.
Consent for publicationNot applicable.
AC and CG declare a patent in association with the University of Palermo - Italy (No.102019000020532 Italian Ministry of Economic Development) related to the content of this manuscript. ACa received honoraria for lectures from Fisher & Paykel, Resmed and Breas not related to the present work. CC received honoraria for lectures from Fisher & Paykel and Resmed not related to the present work. CG received fees for lectures by Philips and received payments by Philips for consultancies in the developing process of the EVO Ventilator and fees for lectures or consultancies from Resmed, Vivisol and Air Liquide not related to the present work.