Tocilizumab is an IL-6 receptor-blocking agent proposed for the treatment of severe COVID-19. The aim of this systematic review was to describe the rationale for the use of tocilizumab for the treatment of COVID-19 and to summarize the available evidence regarding its efficacy and safety.
MethodsMEDLINE, PubMed, EMBASE, pre-print repositories (bioRxiv and medRxiv) and two trial Registries were searched for studies on the use of tocilizumab in COVID-19 or SARS-CoV-2 infection, viral pneumonia, and/or sepsis until 20th June 2020.
ResultsWe identified 3 indirect pre-clinical studies and 28 clinical studies including 5776 patients with COVID-19 (13 with a comparison group, 15 single-arm). To date, no randomized trials have been published. We retrieved no studies at low risk of bias. Forty-five ongoing studies were retrieved from trial registries.
ConclusionsThere is insufficient evidence regarding the clinical efficacy and safety of tocilizumab in patients with COVID-19. Its use should be considered experimental, requiring ethical approval and clinical trial oversight.
The search for the holy grail of medical treatment for COVID-19 (Coronavirus Disease-2019) has led researchers to propose, among other options, the use of IL-6 receptor blocking agents for treatment of symptomatic patients.1 With a death toll of nearly 400.000 worldwide and nearly 7 million known cases2,3 the current lack of targeted effective medication and ongoing reliance on supportive treatment alone remains a major cause of concern.1
Patients with the most severe COVID-19 symptoms (i.e. severe lung damage, septic shock and multiple organ failure) also exhibit what seems to be a hyper-inflammatory syndrome.4 Early serology analyses identified higher IL-6 serum levels in patients with severe COVID-19 (particularly non-survivors) when compared to patients with mild and moderate disease.5,6 As it has been proposed that elevated IL-6 levels may be associated with greater disease severity, the assumption is that they are also associated with worse clinical outcomes7 and vice versa. This assumption underlies the current interest in anti-inflammatory therapies for the treatment of COVID-19.8
One of the IL-6 receptor blocking agents proposed for treatment of COVID-19, tocilizumab, was introduced in the early 2000’s for treatment of autoimmune disorders such as refractory rheumatoid arthritis and systemic juvenile idiopathic arthritis (sJIA)9,10 and has been approved by the FDA since 2017 for treatment of the cytokine release syndrome (CRS) that may occur following some forms of immunotherapy (e.g. CAR-T).11 The aim of this review was to describe the rationale and summarize the available evidence, direct and indirect, regarding the use of tocilizumab for treatment of SARS-CoV-2 infection and to identify and describe ongoing clinical trials with this drug.
MethodsA preliminary search conducted prior to formal study initiation suggested the evidence on the topic is likely to be limited, and yet patients are already receiving the drug, making the issue urgent. Therefore, the protocol of this systematic review was not registered.
PICO questionWe sought information regarding the use of tocilizumab (I) for treatment of COVID-19, SARS-CoV-2 infection, viral pneumonia, and/or sepsis in any population (adults and children) or laboratory model (P) with or without a comparator (C). We aimed to describe any treatment outcome whether solicited or unsolicited (O).
Search methods and article/trial inclusion/exclusion criteriaWe conducted a systematic search of the MEDLINE, PubMed and EMBASE databases from inception to 20th June 2020. We sought pre-clinical and clinical studies addressing the use of tocilizumab for treating COVID-19, SARS-CoV-2 infection, viral pneumonia, and/or sepsis. Our search included the keywords ‘tocilizumab’, ‘covid-19’, ‘coronavirus’, ‘sepsis, ‘pneumonia, and ‘viral infection’ as exact phrases and a combination of subject headings according to databases syntax. We also searched the references of retrieved papers for additional potentially relevant papers. In order to find prepublication manuscripts, we surveyed the pre-print repositories biorRxiv and medRxiv from inception to 20th June 2020 for clinical or pre-clinical studies about the use of tocilizumab in COVID-19 or SARS-CoV-2 infection, viral pneumonia, and/or sepsis. No language restrictions were imposed in any of the searches. Finally, to identify clinical trials studying treatment with tocilizumab for COVID-19, SARS-CoV-2 infection, viral pneumonia, and/or sepsis, we sought trials registered prior to 20th June 2020 in the Chinese Clinical Trial Registry and Clinicatrial.gov. The databases and the trial registries were all screened independently and in duplicate by two of the authors (MI, VG).
In the second stage, the abstracts of all potentially relevant papers and pre-publication manuscripts were screened to identify relevant papers to be downloaded in full. After downloading potentially relevant articles, case reports, case series and reviews were excluded. Discrepancies and doubts regarding the relevance of papers downloaded in full were resolved by discussion and consensus with two additional authors (AC, MG).
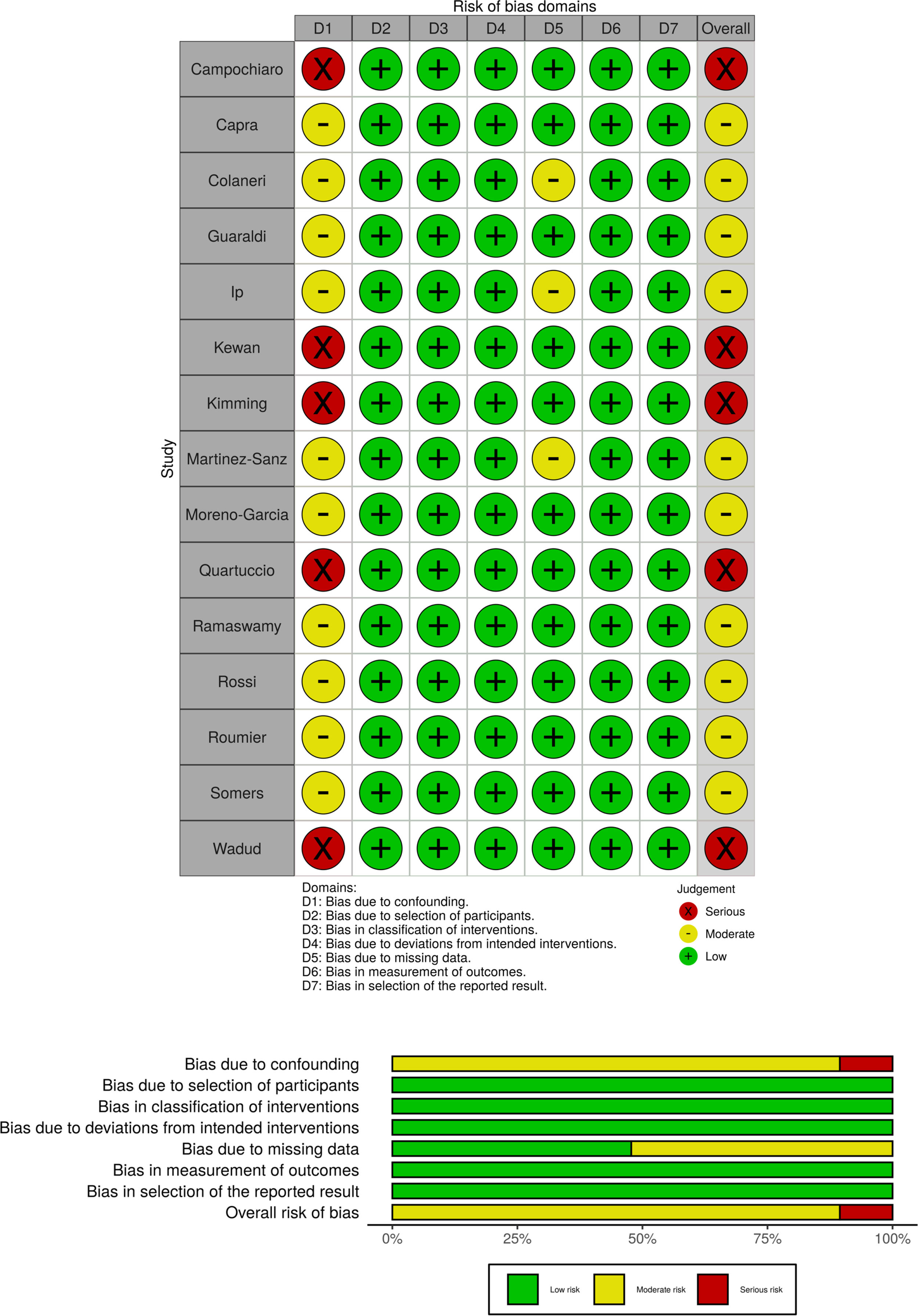
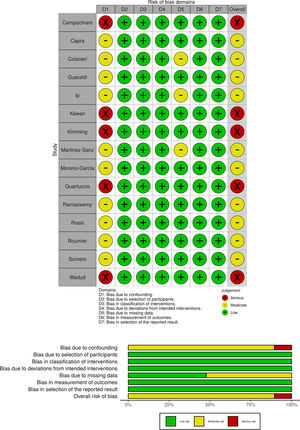
Risk of bias assessmentTwo of the authors (AC, MI) assessed the risk of bias (RoB) of the included studies independently and in duplicate. Disagreements over RoB were resolved by consensus or, if necessary, adjudicated by a third author (AG). The ROBINS-I tool (Risk Of Bias in Non-randomized Studies of Interventions) was used for nonrandomized studies with a comparison group.12 The Newcastle Ottawa Scale (NOS) was used for single-arm nonrandomized studies.13 The domains were rated in accordance with the requirements of the assessment tool used, with the lowest score achieved predominating as accepted. We used the Risk-of-Bias VISualization (robvis) tool14 to present the risk of bias assessments as either a plot or a table.
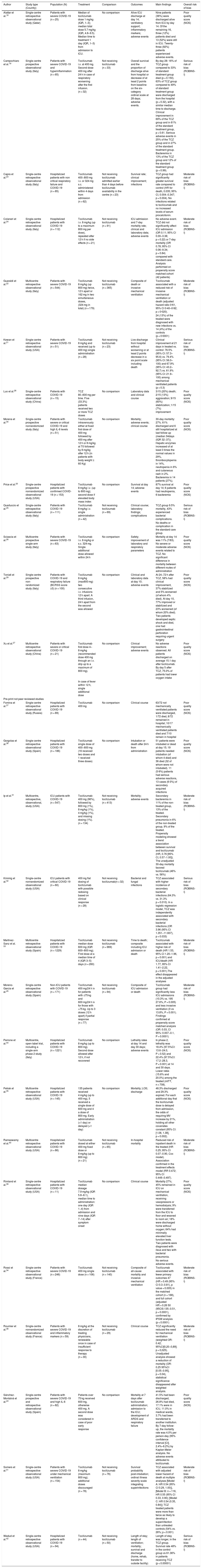
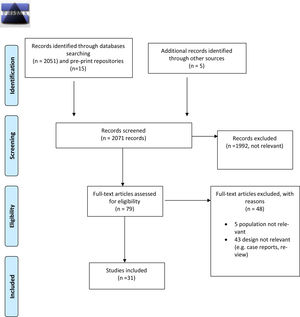
ResultsThe initial search identified 2071 records from MEDLINE, PubMed and EMBASE, pre-print repositories and other sources. After screening of titles and abstracts, removal of duplicates and evaluation of the additional sources retrieved, overall 31 published peer-reviewed papers and preprint non peer-reviewed papers were included (see the PRISMA flow diagram, available in Fig. 1 for the study selection process). Three papers described preclinical studies and 28 papers described clinical studies. The characteristics of the clinical studies included were summarized (see Table 1). In addition, forty-five ongoing clinical studies were retrieved from the trial Registries. These too were tabulated (see Table A1, Appendix A).
Characteristics of the included studies.
| Author | Study type (Country) | Population (N) | Treatment | Comparison | Outcomes | Main findings | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Alattar et al.18 | Single-centre retrospective observational study (Qatar) | Patients with severe COVID-19 (n = 25) | Median of tocilizumab dose 1 mg/kg (IQR, 1–3); median total dose 5.7 mg/kg (IQR, 4.8–9.5). Median time to treatment 1 day (IQR, 1–3) from admission to ICU. | No comparison | Alive ICU discharge at day 14; ventilatory support; inflammatory markers; adverse events | Nine patients (36%) were discharged alive from ICU by day 14. Of the remaining 16, three (12%) patients died and 13 (52%) were still in ICU. Twenty-three (92%) patients experienced adverse events. | Poor quality score (NOS) |
| Campochiaro et al.19 | Single-centre prospective observational study (Italy) | Patients with severe COVID-19 and hyperinflammation (n = 65) | Tocilizumab i.v. at 400 mg. Second dose 400 mg after 24 h in case of respiratory worsening after the first infusion. (n = 32) | Not receiving tocilizumab (n = 33) | Overall survival and the proportion of discharge alive from hospital or decrease of at least 2 points from baseline on the six-category ordinal scale at 28 days; adverse events. | By day 28: 16% of TCZ group compared to 33% of standard treatment group died (p = 0.150). 63% of TCZ group compared to 49% of standard treatment group were discharged from the hospital (p = 0.32), with a similar median time to discharge. Clinical improvement in 69% of the TCZ group and in 61% of the standard treatment group, p = 0.61. Serious adverse events in 25% of the TCZ group and in 27% of the standard treatment group. Bacteremia in 13% of the TCZ group and 12% of the standard treatment group (p = 0.99). | Serious risk of bias (ROBINS-I) |
| Capra et al.23 | Single-centre retrospective observational study (Italy) | Hospitalized patients with non critical respiratory failure and COVID-19 (n = 85) | Tocilizumab 400−800 mg i.v. or 324 mg s.c. administered within 4 days from admission (n = 62) | Not receiving tocilizumab admitted earlier than 4 days before tocilizumab availability in the centre (n = 23) | Survival rate; clinical improvement; infections | TCZ group had significantly greater survival rate compared to control (HR for death, 0.035; 95% CI, 0.004−0.347; p = 0.004). No infections related to tocilizumab and no increased levels of serum procalcitonin. | Moderate risk of bias (ROBINS-I) |
| Colaneri et al.24 | Single-centre retrospective observational study (Italy) | Hospitalized patients with COVID-19 (n = 112) | Tocilizumab i.v. 8 mg/kg (up to a maximum 800 mg per dose), repeated after 12 h if no side effects (n = 21) | Not receiving tocilizumab (n = 91) | ICU admission and 7-day mortality rate; clinical and laboratory data; adverse events | No adverse event. TCZ did not significantly affect ICU admission (OR 0.11; 95% CI 0.00–3.38; p = 0.22) or 7-day mortality (OR 0.78; 95% CI 0.06–9.34; p = 0.84) compared with standard care. Analysis performed on propensity score matched cohort (42 patients) | Moderate risk of bias (ROBINS-I) |
| Guaraldi et al.22 | Multicentre retrospective observational study (Italy) | Patients with severe COVID-19 (n = 544) | Tocilizumab 8 mg/kg (up 800 mg) twice, 12 h apart or 162 mg in two simultaneous doses, (324 mg in total) (n = 179) | Not receiving tocilizumab (n = 365) | Composite of death or invasive mechanical ventilation | Tocilizumab associated with a reduced risk of invasive mechanical ventilation or death (adjusted hazard ratio 0·61, 95% CI 0·40–0·92; p = 0·020). | Moderate risk of bias (ROBINS-I) |
| 24 (13%) of the treated were diagnosed with new infections vs. 14 (4%) of the controls (p < 0·0001) | |||||||
| Kewan et al.21 | Single-centre retrospective observational study (USA) | Patients with severe COVID-19 (n = 51) | Tocilizumab 8 mg/kg and received (up to 400 mg) single administration (n = 28) | Not receiving tocilizumab (n = 23) | Live discharge from hospital without worsening or at least 2 points decrease in a six point scale including death | Clinical improvement at 21 days in treated vs. control: 76.5% (95% CI: 57.3–95.6) vs. 79.4% (95% CI: 56.0–100) and 67.9% (95% CI: 43.2–92.7) vs. 61.9% (95% CI: 21.9–100) among mechanical ventilated patients (p = 0.3) | Serious risk of bias (ROBINS-I) |
| Luo et al.28 | Single-centre retrospective observational study (China) | Patients with COVID-19 (n = 15) | TCZ 80−600 mg per time. Five (33.3%) patients received two or more TCZ doses | No comparison | Laboratory data and clinical course | 3/15 (20%) death; 2/15 (13%) aggravation; 9/15 (60%) stabilization; 1/15 (7%) improvement | Poor quality score (NOS) |
| Morena et al.30 | Single-centre prospective nonrandomized study (Italy) | Patients with severe or critical COVID-19 and high IL-6 levels (n = 51) | Tocilizumab intravenously either at fixed first dose of 400 mg followed by 400 mg after 12 h or 8 mg/kg at T0 followed by 8 mg/kg after 12 h (in patients with body weight ≥ 60 Kg) | No comparison | Mortality; adverse events; clinical course | 30-day mortality: 27%. 61% discharged and 6 still hospitalized at last follow-up (median 34days (IQR 32−37)). Hepatic enzymes increased of at least 3 times the normal values in 29%, thrombocytopenia in 14%, neutropenia in 6% and cutaneous rash in 2%. Bacteremia in 14 patients (27%). | Poor quality score (NOS) |
| Price et al.20 | Single-centre prospective nonrandomized observational study (USA) | Hospitalized patients with confirmed COVID-19 (n = 153) | Tocilizumab 8 mg/kg i.v. (up to 800 mg); second dose if elevated body mass index | No comparison | Survival at day 14; adverse events | 87% survival at day 14; 6 patients had neutropenia, 6 bacteremia | Poor quality score (NOS) |
| Quartuccio et al.25 | Single-centre retrospective observational study (Italy) | Patients with COVID-19 (n = 111) | Tocilizumab 8 mg/kg i.v. single administration (n = 42) | Not receiving tocilizumab (n = 69) | Clinical course; laboratory findings; complications | TCZ group 9.5% mortality, 43% experienced bacterial complications. | Serious risk of bias (ROBINS-I) |
| No deaths or complication in the standard care group. | |||||||
| Sciascia et al.26 | Multicentre prospective single-arm study (Italy) | Patients with severe COVID-19 (n = 63) | Tocilizumab i.v. 8 mg/kg or s.c. 324 mg. Single additional dose allowed within 24 h | No comparison | Safety; improvement of laboratory and respiratory parameters | Mortality at day 14 was 11% (7/63). No severe or moderate adverse events related to TCZ. No significant difference in mortality between different routes of administration | Poor quality score (NOS) |
| Toniati et al.29 | Single-centre prospective non-randomized study (Italy) | Patients with COVID-19 and respiratory failure (BCRSS score ≥3) (n = 100) | Tocilizumab 8 mg/kg (max800 mg) two consecutive i.v. infusions 12 h apart. A third infusion, 24 h apart from the second was allowed | No comparison | Clinical and laboratory data at day 10; adverse events | At 24−72 h after TCZ, 58% had clinical improvement, 37% stabilized and 5% worsened (of whom 4% died). At day 10, 77% improved or stabilized and 23% worsened (of whom 20% died). Two patients developed septic shock and died, one had gastrointestinal perforation requiring urgent surgery | Poor quality score (NOS) |
| Xu et al.27 | Multicentre retrospective observational study (China) | Patients with severe or critical COVID-19 (n = 21) | Tocilizumab first dose 4–8 mg/kg (recommended dose 400 mg through an i.v. drip up to a maximum of 800 mg). | No comparison | Clinical improvement; adverse events | No adverse reactions observed. All patients discharged on average 15.1 day after tocilizumab. By day 5 after TCZ, 75.0% of patients had lower oxygen intake | Poor quality score (NOS) |
| In case of fever within 12 h, single additional dose | |||||||
| Pre-print not peer reviewed studies | |||||||
| Fomina et al.37 | Single-centre retrospective observational study (Russia) | Hospitalized patients with COVID-19 (n = 89) | Tocilizumab 400 mg | No comparison | Clinical course | 63/72 not mechanically ventilated patients were discharged, 1/72 died, 8/72 remained in hospital; 10/17 mechanically ventilated patients died and 7/10 remain in hospital | Poor quality score (NOS) |
| Gorgolas et al.36 | Single-centre retrospective observational study (Spain) | Hospitalized patients with COVID-19 (n = 186) | Tocilizumab single dose of 400−600 mg (16 received two doses and 1 received three doses) | No comparison | Intubation or death after 24 h from administration | 51 patients were intubated or dead at day 15; 19 patients needed intubation (of whom 4 died) and 36 died (32 of whom were not intubated). 11 (5·9%) patients had serious adverse reactions, 13 cases (6·3%) of secondary acquired infections | Poor quality score (NOS) |
| Ip et al.31 | Multicentre, retrospective, observational, study (USA) | ICU patients with COVID-19 (n = 547) | Tocilizumab: 400 mg (96%), followed by 800 mg (1%), 8 mg/kg (1%), 4 mg/kg (1%), and missing dosing (1%).(n = 134) | Not receiving tocilizumab (n = 413) | Mortality; adverse events | Secondary bacteremia in 11% of the non-treated group, 13% of the treated. Secondary pneumonia in 6% of the non-treated group, 9% of the treated. Propensity modeling showed a trend association between survival and tocilizumab (HR, 0.76 [95% CI, 0.57−1.00]). The unadjusted 30-day mortality favored tocilizumab (46% vs. 56%) | Moderate risk of bias (ROBINS-I) |
| Kimmig et al.32 | Single-centre nonrandomized observational study (USA) | ICU patients with critical COVID-19 (n = 60) | 400 mg flat dosing of tocilizumab with possible redosing based on clinical response (n = 28) | Not receiving tocilizumab(n = 32) | Bacterial and fungal infections | TCZ associated with higher incidence of secondary bacterial infections (64.3% vs. 31.3% p = 0.010). In a logistic regression model, TCZ was independently associated with secondary bacterial infections (OR 3.96 (95% CI 1.351−11.607), p = 0.033) | Serious risk of bias (ROBINS-I) |
| Martinez-Sanz et al. 33 | Multicentre retrospective observational study (Spain) | Hospitalized patients with COVID-19 (n = 1229) | Tocilizumab median dose 600 mg (IQR 600–800 mg). First dose at a median time of 4 (IQR 3–5) days (n = 260) | Not receiving tocilizumab (n = 969) | Time to death; composite including ICU admission or death | Tocilizumab associated with higher risk of death (HR 1.53, 95% CI 1.20–1.96, p = 0.001) and ICU/death (HR 1.77, 95% CI 1.41–2.22, p < 0.001).The effect disappeared in the adjusted analyses | Moderate risk of bias (ROBINS-I) |
| Moreno-Garcia et al.45 | Single-centre retrospective observational study (Spain) | Non-ICU patients with COVID-19 (n = 171) | Tocilizumab 400 mg/24 h iv for patients with ≤75 kg and 600 mg/24 h iv for those with >75 kg. Up to 3 doses (12 h apart) if partial response (n = 77) | Not receiving tocilizumab (n = 94) | Composite of ICU admission or death | Tocilizumab group had significantly less ICU admissions (10.3% vs. 195 27.6%, P = 0.005) and less invasive ventilation (0 vs 13.8%, P = 0.001). Findings confirmed at propensity score matched analysis (OR: 0.03, CI 95%: 0.007−0.1, P = 0.0001) | Moderate risk of bias (ROBINS-I) |
| Perrone et al.44 | Multicentre, open-label trial, including a single-arm phase 2 study (Italy) | Hospitalized patients with COVID-19 (n = 1221) | Tocilizumab 8 mg/kg (up to 800 mg). Second dose allowed after 12 h, if not recovered | No comparison | Lethality rates at day 14 and day 30 days; adverse events | In phase 2, lethality was 18.4% (97.5%CI: 13.6−24.0, P = 0.52) and 22.4% (97.5%CI: 17.2−28.3, P < 0.001) at 14 and 30 days. Lower rates (15.6% and 20.0%) among the treated (mITT, n = 708). | Poor quality score (NOS) |
| Petrak et al.35 | Multicentre retrospective observational study (USA) | Hospitalized patients with COVID-19 (n = 145) | 135 patients received 4 mg/kg (up to 400 mg), 5 received a single dose of 600 mg and 4 a dose of 800 mg. Early administration (<1 day) or delayed (>1 day) | No comparison | Mortality; LOS; discharge | 48.3% discharged and 29.3% expired. For each additional day that the tocilizumab dose is delayed from admission, the odds of requiring MV increase by 21%, holding all other covariates constant (95% CI: [1.08, 1.38], p = 0.002). | Poor quality score (NOS) |
| Ramaswamy et al.40 | Multicentre retrospective observational study (USA) | Hospitalized patients with COVID-19 (n = 86) | Tocilizumab dosed at either 400 mg fixed dose or 8 mg/kg (up to 800 mg) (n = 21) | Not receiving tocilizumab (n = 65) | In-hospital mortality | Reduced risk of inpatient death in the treated (HR 0.25; 95% CI 0.07−0.90, Cox model). Association confirmed in the treatment effects model (RR 0.472; 95% CI 0.449−0.497). | Moderate risk of bias (ROBINS-I) |
| Rimland et al.39 | Single-centre retrospective observational study (USA) | Hospitalized patients with COVID-19 (n = 11) | Tocilizumab median dosage 7.9 mg/kg (IQR 5.8−8.1), median time to administration: one day (IQR 1−4) from admission and nine days (IQR 7−14) after symptom onset | No comparison | Clinical course | Mortality 27%; 45% remained in ICU on mechanical ventilation, receiving vasopressors or hemodialysis; 9% were transferred from the ICU to floor and weaned to room air; 18% were discharged home without oxygen; 64% had minimally elevated liver function tests. Two patients were diagnosed with ileus and two with bacterial pneumonia. | Poor quality score (NOS) |
| No serious adverse events. | |||||||
| Rossi et al.34 | Single-centre retrospective observational study (France) | Patients with severe COVID-19 (n = 246) | Tocilizumab 400 mg single dose (n = 106) | Not receiving tocilizumab (n = 140) | Composite of all-cause mortality and invasive mechanical ventilation | Tocilizumab associated with fewer primary outcomes 47 (HR = 0.49 (95% CI 0.3−0.81), p value = 0.005) in the matched cohort (n = 168), and full cohort (adjusted HR = 0.26 50 (95CI0.135−0.51, p = 0.0001), confirmed by IPSW analysis (p < 0.0001) | Moderate risk of bias (ROBINS-I) |
| Roumier et al.38 | Single-centre nonrandomized observational study (France) | Patients with severe COVID-19 and inflammatory markers (n = 59) | 8 mg/kg at the discretion of treating physicians, renewable once in case of insufficient response to therapy (n = 30) | Not receiving tocilizumab (n = 29) | Clinical course | TCZ significantly reduced the need for mechanical ventilation (weighted OR: 0.42; 95%CI[0,20−0,89]; p = 0,025). Unadjusted analysis showed a reduction of mortality (OR: 0.25 95%CI [0.05−0.95], p = 0.04), statistical significance disappeared after weighted analysis. | Moderate risk of bias (ROBINS-I) |
| Sánchez-Montalvá et al.43 | Single-centre prospective and retrospective observational study (Spain) | Patients with severe COVID-19 and high IL-6 (n = 82) | Patients over 75 kg received 600 mg, otherwise 400 mg. A second dose was considered in case of poor early response | No comparison | Mortality at 7 days after tocilizumab administration; admission to the ICU; development of ARDS and respiratory failure | 41.5% had been discharged, 26.8% had died, 17.1% were in ICU, 11.0% in medical wards, 3.7% had been transferred to another institution. By 7-day follow-up, the mortality rate was 4.0% per person-day (95% confidence interval [CI], 2.4%–6.2%) by Kaplan-Meier analysis. No adverse events attributed to tocilizumab. | Poor quality score (NOS) |
| Somers et al.41 | Single-centre retrospective observational study (USA) | Patients with severe COVID-19 under mechanical ventilation (n = 154) | Tocilizumab 8 mg/kg (maximum 800 mg); additional doses discouraged (n = 78) | Not receiving tocilizumab (n = 76) | Survival probability post-intubation; ordinal illness severity scale integrating superinfections | TCZ associated with adjusted lower hazard of death at multiple analyses [Model A: HR 0.54 (95% CI 0.29, 1.00)], [Model B: n = 116, HR 0.55 (95% CI 0.33, 0.90); [Model C: HR 0.54 (0.35, 0.84)]; TCZ treated patients were more than twice as likely to develop a superinfection than untreated controls (54% vs. 26%; p < 0.001) | Moderate risk of bias (ROBINS-I) |
| Wadud et al.42 | Single-centre retrospective observational study (USA) | Hospitalized patients with COVID-19 (n = 94) | Tocilizumab (n = 44) | Not receiving tocilizumab (n = 50) | Length of stay; length of ventilation; mortality; survival and discharge (home, rehab, transfer to outside facility) | Length of stay was longer, in the TCZ group. Survival rate 48% in the control group vs 61.36% in patients receiving TCZ (p < 0.00001) | Serious risk of bias (ROBINS-I) |
The table shows the main characteristics of the included studies.
ARDS, acute respiratory distress syndrome; BCRSS, Brescia COVID-19 Respiratory Severity Scale; CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; mITT, modified intention to treat; OR, Odds ratio; SARS, severe acute respiratory syndrome; TCZ, tocilizumab; USA, United States of America.
The hypothetical justification for treating patients with tocilizumab in the context of COVID-19 stems from indirect findings. These include several pre-clinical studies describing a beneficial effect in cellular and murine models of sepsis and influenza.15–17
In a cell model of sepsis (Human monocyte cell line THP-1), tocilizumab reduced the expression of TNF and IL-10, down-regulated inflammasome activation (reduced levels of NLRP3 and CASP1) and inhibited monocyte phagocytic activity. The authors of the study suggested that if suppressing the ‘cytokine storm’ is important when treating sepsis, these effects may be beneficial.15
Tocilizumab has also been evaluated in a murine model of Influenza A virus infection. Mice were anesthetized, intubated, and infected with mouse-adapted H1N1. The tocilizumab treated group (8 mg/kg 24 h before infection) and the controls were compared. Reduced skeletal muscle weakness (measured as digital grip strength), preserved muscle weight and increased short-term and long-term mortality were registered in the treated group, in comparison with controls. The mice manifesting distress were sacrificed and their deaths were also recorded as mortality.16
In a rat model of sepsis-induced acute lung and kidney injury, tocilizumab (4−8 mg/kg) reduced mortality. The authors also observed normalization of persistently high serum levels of IL-6 in septic rats after treatment with tocilizumab and improved lung wet/dry weight ratio and total protein content in the treatment group, in comparison with the sham group.17
Clinical studiesWe retrieved 13 published clinical studies18–30 and 15 pre-print (i.e. pre peer review) clinical studies,31–45 respectively evaluating 1396 and 4380 patients, for a total of 5776 patients. The main characteristics of the included clinical studies are presented in Table 1. Only three peer reviewed papers22,26,27 and five prepublications31,33,35,40,44 were multicenter studies. Thirteen studies included more than 100 patients, but in these studies the number of patients receiving tocilizumab was not large. One published trial included 112 patients but only one in five patients (n = 21) received tocilizumab.24 Another published trial included 111 patients among whom less than half (n = 49) were treated.25 One prepublication included 547 patients of which a third (n = 134) were treated31 and another included 1229 patients of which 260 received tocilizumab.33 Seven of the 13 published papers and six of the 15 prepublications presented no comparator.
The risk of bias assessments are shown in Fig. 2 (nonrandomized studies with comparison) and in Appendix A, Table A2 (nonrandomized single-arm studies). Ten studies were at moderate and five at serious risk of bias (ROBINS-I); thirteen studies were judged to be of “poor quality” (NOS). None of the studies were assessed as having a low risk of bias.
DiscussionThe mechanism of action and pharmacological properties of tocilizumabTocilizumab is a humanized monoclonal antibody capable of interfering with the IL-6 soluble and membrane binding site of the receptor (IL-6R), thereby blocking the assembling of the activated complex with the transmembrane protein (gp130-IL-6-sILr). Tocilizumab is also able to block IL-6 trans-signaling46 which is strongly related to the pro-inflammatory effects of IL-6 (e.g. release of acute phase proteins). Tocilizumab has a non-linear pharmacokinetic profile, with a dose-response curve that plateaus at an approximate dose of 800 mg.46 The half-life of tocilizumab is dose-dependent and comparable to the half-life of IgG1.47
Interleukin-6 (IL-6) and COVID-19IL-6 is a pleiotropic cytokine secreted by neutrophils, monocytes and macrophages and involved in the inflammatory response. It has a soluble (sIL-6R) and a membrane binding site (mIL-6R), constituting its receptors. IL-6 can bind its mIL-6R at low doses or, at higher doses, its sIL-6R (trans-signaling), creating the activated complex with gp130 protein.48 Signaling is mediated by Janus kinases (JAK) and Ras/mitogen-activated protein kinase (MAPK)/NF-κB-IL-6.48 IL-6 promotes B and T cells differentiation, acute phase protein production and osteoclast activation.46 High levels of IL-6 have been listed among the main features of cytokine storm and cytokine release syndrome (CRS), both of which are characterized by an exaggerated release of pro-inflammatory cytokines and potentially life-threatening multiorgan damage.48 IL-6 also seems to be involved, through these mechanisms, in the pathophysiology of COVID-19, especially in the most severe forms of the disease.5,6,49,50 Furthermore, elevated levels of IL-6 have been associated with a hypercoagulable state in both animals and humans,51,52 and coagulopathy is another characteristic of patients with COVID-19 at high risk of death.53
Evidence on efficacy and safety of tocilizumab for COVID-19The current systematic review shows that although indirect preclinical data suggests rationale for using tocilizumab and observational studies suggest that treatment with tocilizumab may be associated with more favorable outcomes compared to standard care in patients with severe or critical COVID-19, up till now no RCTs have been published or made available pre-print regarding either the effectiveness or the safety of tocilizumab in the context of COVID-19.
The effects of tocilizumab against IL-6 related pro-inflammatory and pro-coagulant status53 partially explain its potential role in COVID-19.54 However, it is worth remembering that there is yet no evidence that suppressing the physiological inflammatory response to the virus is indeed beneficial. Previous experience with pharmaceutic inflammatory response modulation in patients with ARDS55 and sepsis56 have been notoriously unsuccessful.
Several observational studies have examined whether tocilizumab has any effect in patients with COVID-19. Although many of the patients included in these studies had severe or critical disease and many were admitted to ICUs, drawing conclusions from the findings of these studies is a leap of faith, given that most had small sample sizes and high or moderate risk of bias, mostly due to confounding. The identified studies also varied in dosing (single or double), and drug availability issues emerged in some centres, which may have influenced both sample sizes and study designs. Finally, the literature suggests the presence of two phases in the pathophysiology of COVID-19. An early phase characterized by a high viral load and limited systemic impairment, and a late phase, with elevated cytokine levels and a hyperinflammatory state.4 Regarding the timing of drug administration, that could therefore potentially determine treatment outcomes, a pre-print study evaluated the effect of early vs late administration of the drug on the outcomes, but the association remains unclear and understudied.35 The authors found that for each additional day of delay from the admission to tocilizumab administration, the odds of receiving mechanical ventilation independently increase by 21% (95% CI: [1.08, 1.38], p = 0.002).35
Clinical studies on patients with COVID-19 have also evoked some safety concerns. The risk of secondary infection complications is unclear. Among the studies with a comparison group, six found a higher rate of infections among treated, compared to untreated, patients.19,22,25,31,32,41 Hepatotoxic effects, neutro- and thrombocytopenia and intestinal perforation have also been described.57–59 This is worrying, particularly given that data from a clinical study promoted by the Italian agency for medicines (Agenzia Nazionale del Farmaco - AIFA) suggests no significant benefit in terms of either mortality (3.3% vs. 3.2%) or ICU admission (10.0% vs 7.9%) in patients not requiring mechanical respiratory support, when treated with tocilizumab vs. controls.60
Recommendations ad ongoing studiesSeveral clinical practice guidelines already include tocilizumab as a therapeutic option for COVID-19. The most recent Chinese guideline61 suggests tocilizumab, at a dose of 4−8 mg/kg, for patients with extensive lung lesions and for severe cases with increased levels of IL-6 or with complications.61 The Italian Society of Infectious and Tropical disease (SIMIT - Lombardy section),62 recommends selection of patients who have recovered from the initial high viral load (i.e. afebrile from >72 h or with symptoms for >7 days), those with high IL-6 levels (>40 pg/mL), those with increasing levels of D-dimer, C-reactive protein, ferritin or fibrinogen levels and those needing ventilation support (CPAP, NIV or IMV) for treatment with tocilizumab. However, both the ‘Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) and the U.S. National Institutes of Health (NIH) state that the evidence does not suffice to issue recommendations regarding the use of tocilizumab for COVID-19.63,64 The World Health Organization (WHO)65 and the Infectious Diseases Society of America (IDSA)66 both recommend that tocilizumab only be administered within the context of a clinical trial.
Our search identified 45 registered ongoing trials, including 18 multicentre randomized trials in several countries across 4 continents. The details of the studies underway are provided in Appendix A (Table A1). The results of these trials will hopefully provide sufficient data regarding the role of tocilizumab in the context of COVID-19.
ConclusionAlthough basic science suggests rationale for administration of IL-6 receptor antagonists to patients with COVID-19, the clinical evidence regarding the efficacy and safety of tocilizumab for COVID-19 remains observational only and is methodologically flawed. As concerns have also been raised regarding the possibility of secondary infection, the use of this drug should be limited to the context of a clinical trial and accompanied by ethical committee approval and/or informed consent, as well as appropriate monitoring for side effects.
FundingNone.
Conflicts of interestAll authors declare to have no competing interests.
Ethics approvalNot applicable.
Consent to participateNot applicable.
Consent for publicationNot applicable.
Availability of data and materialAvailable.
Code availabilityNot applicable.
Authors' contributionsAC conceived the content, drafted the manuscript, approved the final version to be submitted. MI, MG, VG, AP helped in writing the manuscript and revised it critically for important intellectual content, approved the final version to be submitted. CG, AG, SE, MC conceived the content, drafted the manuscript, approved the final version to be submitted.
None.