Silicosis mostly happens in workers with high silica exposure and may accompany the development of various diseases like tuberculosis, cancer, or autoimmune diseases. The term silico-tuberculosis describes a condition in which an individual is affected by both silicosis and tuberculosis at the same time. This systematic review and meta-analysis study was conducted to evaluate the risk of tuberculosis in silicosis patients and individuals exposed to silica dust.
MethodsWe performed a systematic search for relevant studies up to 6 September 2022 using PubMed/ Medline, and Embase with the following keywords in titles or abstracts: “silicosis” OR “silicoses” OR “pneumoconiosis” OR “pneumoconioses” AND “tuberculosis”. Cohort and case-control studies containing relevant and original information about tuberculosis infection in silicosis patients were included for further analysis. Pooled estimates and 95% confidence intervals (CI) for the relative risk of tuberculosis in individuals with silicosis compared to those without; these were evaluated using the random effects model due to the estimated heterogeneity of the true effect sizes.
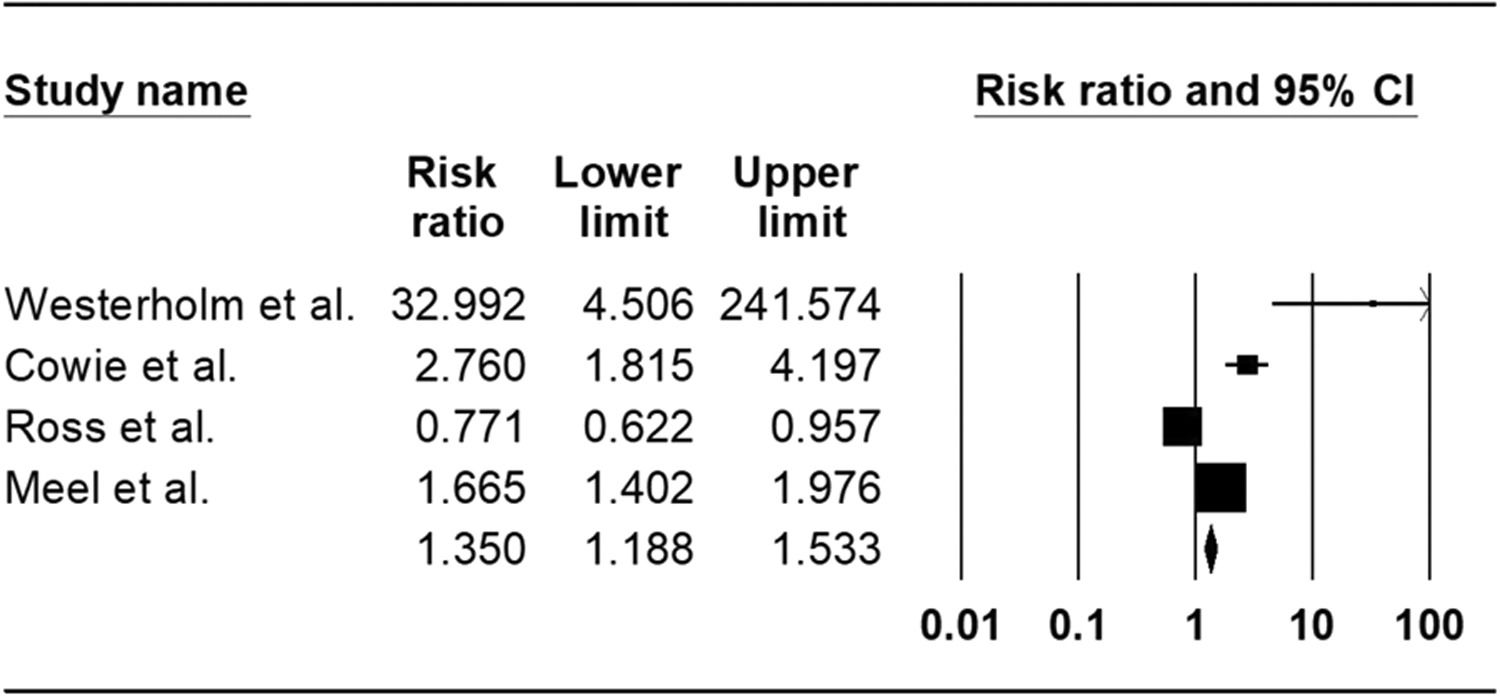
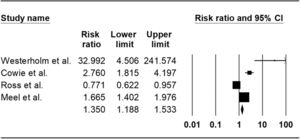
ResultsOut of 5352 potentially relevant articles, 7 studies were eligible for systematic review, of which 4 cohort studies were included for meta-analysis. The total population of all studies was 5884, and 90.63% were male. The mean age of participants was 47.7 years. Our meta-analysis revealed a pooled risk ratio of 1.35 (95%CI 1.18-1.53, I 2: 94.30%) which means an increased risk of silicosis patients and silica-exposed individuals to tuberculosis infection.
ConclusionSilicosis and silica dust exposure increase the risk of tuberculosis. Therefore, we suggest that individuals with long-time silica exposure, like mine workers, be routinely considered for both silicosis and tuberculosis screening programs.
Silicosis is an occupational lung disease caused by chronic exposure to silica dust, mainly composed of quartz dust, a human carcinogen.1 Silicosis is caused by the deposition of silica particles in the lower respiratory tract leading to chronic inflammation, collagen deposition, and fibrotic lesions, which may vary from subclinical pathological changes to severe lung tissue damage and reduce life expectancy.2,3 Although silicosis may be fatal and irreversible, it can be prevented. 4 It mainly affects industrial workers with prolonged and intensive exposure, such as coal miners,2 and it is prevalent in low-income countries with annual global mortality higher than 10,000 people.5 Although its notification rate is high, several cases are not diagnosed.6
There is not a specific diagnostic test for silicosis, however, occupational history and chest radiological abnormalities (i.e., nodular opacity) can confirm the diagnosis. 7
Silicotuberculosis is a condition characterized by the co-existence of silicosis and tuberculosis (TB). The incidence of tuberculosis in patients with silicosis is 21.8 times higher than that recorded in the general population.8 The most important risk factors for developing silicotuberculosis are: exposure to crystalline silica and sandblasting and its duration, age (>30 years), being male, HIV positive, smoking, chronic obstructive pulmonary disease, being migrant from endemic countries, and silicosis severity. 9
High silica impairs the activity of alveolar macrophages, leading to apoptosis and susceptibility to mycobacterial infection.9-13 After inhalation of silica particles, they enter the alveolar space and interact with macrophages, and then, they sink into the phagosome. A macrophage receptor with a collagenous structure (MARCO), is a Class A protein scavenger receptor on the surface of macrophages that intercedes binding and ingestion of unopsonized environmental particles such as TiO2, Fe2O3, silica, and other nano-sized substrates. MARCO has been described as the main molecule responsible for the recognition and uptake of silica and there will be no particle uptake or cell death in the absence of this recepeptor.14,15 Some pattern recognition receptors, such as Toll-like receptors (TLRs), play an essential role in responding to bacterial infections. Significant downregulation of TLR2 following silica exposure may contribute to higher TB susceptibility.16 Genetic polymorphisms of tumor necrosis factor-alpha (TNF-α), natural resistance-associated macrophage protein 1, and inducible nitric oxide synthase (iNOS) of macrophages were shown to affect the response to exposure to silica.17,18
This systematic review and meta-analysis study was conducted to evaluate the risk of tuberculosis in silicotic patients and individuals exposed to silica dust.
MethodsThis review conforms to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (PROSPERO pending ID: 388636).19 Also, the PRISMA 2020 item checklist is available in supplementary 1.
Search strategyWe searched Pubmed/Medline, and Embase, for studies reporting the association between silicosis and pulmonary TB published up to 6 September 2022. There was no specific limitation for the starting date of search. The following search strategy was used: (“silicosis”[MeSH Terms] OR (“silicosis”[Title/Abstract] OR “silicoses”[Title/Abstract] OR “pneumoconiosis”[Title/Abstract] OR “pneumoconioses”[Title/Abstract]) OR “pneumoconiosis”[MeSH Terms]) AND (“tuberculosis”[MeSH Terms] OR “tuberculosis”[Title/Abstract]).
Study selectionThe records found through database searching were merged, and the duplicates were removed using EndNote X8 (Thomson Reuters, New York, NY, USA). Two reviewers (MA and BM) independently conducted title and abstract screening to exclude irrelevant studies. Two other reviewers (MA and BM) retrieved and assessed the full text of potentially eligible records. Inclusion criteria were: populations exposed to silica and silicosis patients; studies reporting comparative effect estimates, specifically case-control or cohort studies reporting relative risks or odds ratios across groups with and without silicosis (including different grades). Only English-medium papers were considered.
Studies without comparison between those with and without the disease, studies focused on latent TB infection, cross-sectional and mortality studies, and autopsy-based studies were excluded. Review articles, duplicate publications, conference abstracts, editorials, postmortem studies, and those in which the full text of papers was not found were also excluded.
Data extractionThe data extraction form was designed by PJ and the following data were extracted by BD, BM, MA, and FK: first author's name, type of study, year of publication, study period, the country/ies where the study was performed, study population, age, gender, occupation, mentioned confounding factors, diagnostic approach for silicosis and tuberculosis, duration and grade of silicosis, follow-up duration and technique, the estimated effect measures of silicosis and TB, and the total number of following items: TB population, silicosis population, TB population with and without silicosis, silicosis without TB, and the total number of non-silicosis individuals.
Quality assessmentThe Newcastle-Ottawa Scale (NOS) for observational studies was used to perform a study quality assessment.20 The NOS scale assesses the risk of bias in observational studies with three domains: 1) selection of participants, 2) comparability, and 3) outcomes. A study could be given a maximum of one point for each numbered item within the selection and outcome areas, and a maximum of two points allocated for comparability. The low, moderate, and high-quality studies were given scores of 0–3, 4–6, and 7–9, respectively.
Data synthesis and analysisStatistical analyses were conducted using Comprehensive Meta-Analysis software, version 2.0 (Biostat Inc., Englewood, NJ, USA). Pooled estimates and 95% confidence intervals (CI) for the relative risk of TB in silicosis individuals compared to those without silicosis ones were evaluated using the random effects model due to the estimated heterogeneity of the true effect sizes. The between-study heterogeneity was assessed by Cochran's Q and the I2 statistic. Publication bias was evaluated using Begg's tests (P < 0.05 was indicative of a publication bias) and forest plot.
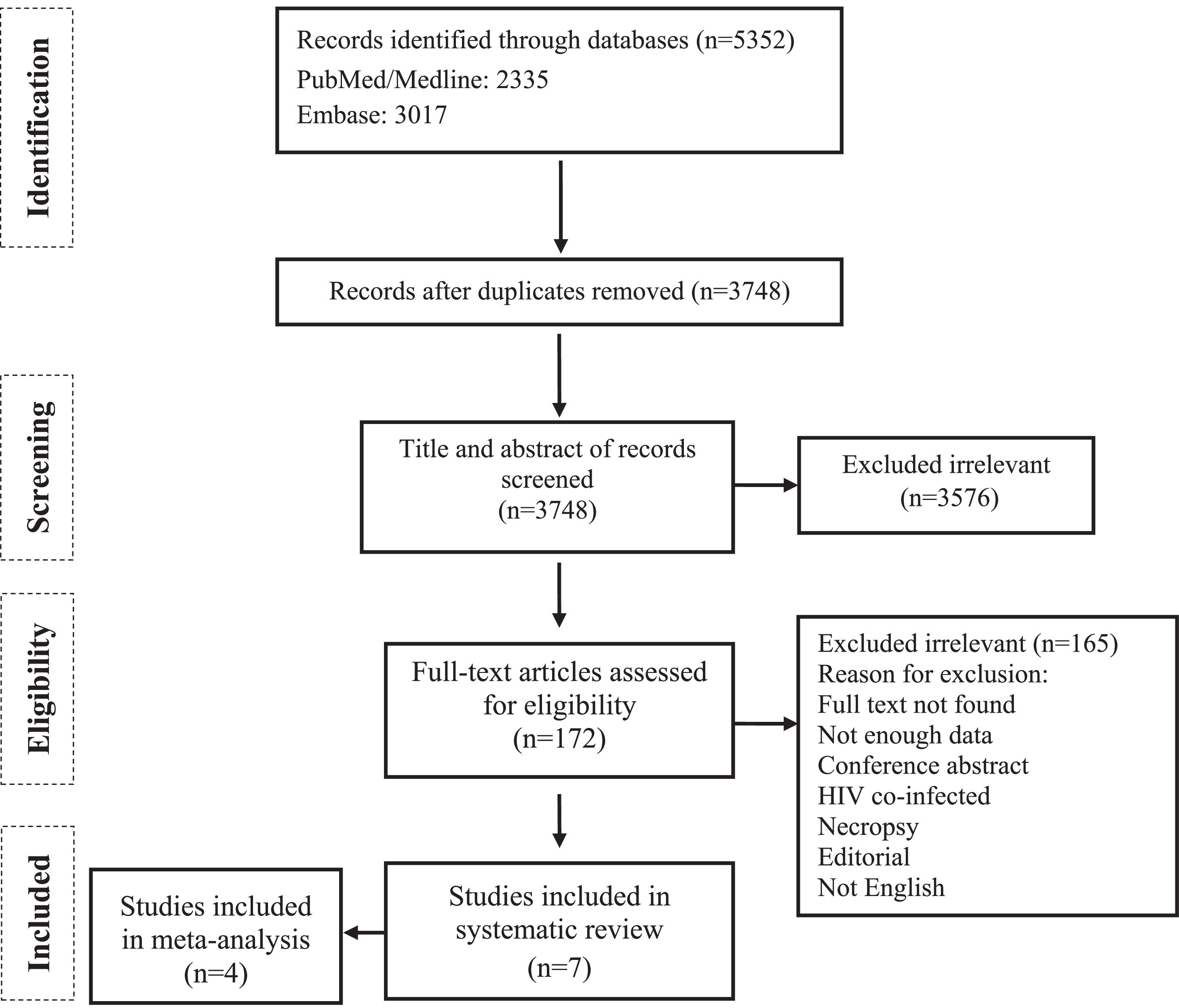
ResultsThe primary search identified a total of 5,352 potentially relevant publications. Removing 1,604 duplicates, 3,748 records were screened, and 172 full-text articles were further assessed for eligibility. Finally, 7 studies met the inclusion criteria and were selected for qualitative analysis,21-27 of which 4 cohort studies21-24 were included for further meta-analysis (Fig. 1).
Based on the Newcastle-Ottawa Scale (NOS), which was used to assess the quality of the cohort studies, the mean (standard deviation [SD]) NOS score was 7.5 (1.29), which is suggestive of a high methodological quality and a low risk of bias of the included studies (Table 1).
Quality assessment of the cohort studies included in the meta-analysis (The NOS tool)20
| Author | Selection | Comparability | Outcome | Total quality score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort | Selection of non-exposed Cohort | Ascertainmentof exposure | Demonstration that outcomeof interest was not present at the start of the study | Adjust for the most important risk factors | Adjust for other risk factors | Assessment of outcome | Follow-up length | Loss to follow-up rate | ||
| Westerholm et al.24 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Cowie et al.21 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Ross et al.23 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Meel et al.22 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
NOS: The Newcastle-Ottawa Scale
Five articles described cohort studies21,23-25,28 and two case-control studies.26,27 Publication dates ranged from 1984 to 2013. Most of the studies were conducted in South Africa (n= 4, 57.14%),21-23,26 one (14.28%) in Sweden,24 one (14.28%) in Hong Kong, 25 and one (14.28%) in Iran. 27 The total population was 5,884 persons. The male gender was dominant (n=5,333, 90.63%), and the mean age was 47.70 years (Table 2 and Table 3).
Characteristics of the included studies.
| Authors | Type of Study | Year of publication | Study time | Country | Silicosis diagnosis method | TB diagnosis method | Follow-up duration | Follow-up technique |
|---|---|---|---|---|---|---|---|---|
| Westerholm et al.24 | Retrospective Cohort | 1984 | 1959-1980 | Sweden | CXR | CXR + Sputum smear /culture | 20 years | CXR |
| Cowie et al.21 | Cohort | 1994 | 1984-1991 | South Africa | CXR | Sputum smear and culture (3 times), CXR, Mantoux test with 5TU, Tuberculin skin test, and when indicated, histologic examination | 7 years | Clinical evaluation, 100-mm CXR |
| Corbett et al.26 | Case-control | 1999 | 1975-1990 | South Africa | CXR | Sputum smear and culture and fluorescent microscopy | NA | NA |
| Chang et al.25 | Cohort | 2000 | 1988-1999 | Hong Kong | Pneumoconiosis Medical Board (PMB) consists of a consultant chest physician, a senior medical officer, and an occupational health officer. | One of these criteria: 1) two or more positive cultures for Mycobacterium tuberculosis; 2) one positive culture for M. tuberculosis plus compatible clinical history and radiological picture; 3) one or more positive smears for acid-fast bacilli plus compatible clinical history and radiological picture; 4) a compatible clinical history and a radiological picture or histological evidence plus improvement after anti-tuberculosis treatment. | 95.00 ± 2.44 months | Clinical evaluation, radiologic imaging, and sputum examination |
| Ross et al.23 | Retrospective Cohort | 2010 | 1995-2000 | South Africa | Mass Miniature Radiography (ILO Classification System) | Sputum smear and culture (3 times) | 3 years | Radiological screening for TB + PFT + a questionnaire eliciting respiratory, smoking, and occupational information |
| Meel et al. 22 | Retrospective Cohort | 2013 | 1997-1999 | South Africa | CXR & physical examination | CXR & physical examination | NA | NA |
| Yarahmadi et al.27 | Case-control | 2013 | 2006-2011 | Iran | CXR, medical & occupational history, and physical examination, consultation with a pulmonologist | Three sputum smears with or without sputum culture | NA | Clinical evaluation, radiologic imaging, and sputum examination |
NA: Not Available; PFT: Pulmonary Function Test; CXR: Chest X-Ray.
Characteristics of the study population.
| Authors | Study population | Mean age | Gender | Occupation | Mentioned confounding factors | Grade of silicosis | Duration of silicosis History | Correlation between silicosis & TB | Estimate (95% CI) (silicosis vs. non-silicosis) |
|---|---|---|---|---|---|---|---|---|---|
| Westerholm et al.24 | 1522 (Exposed:712, non-exposed: 810) | NA | 1522 M | Mining, Quarrying, tunneling, and steel and iron foundries | Occupation, age at first exposure to silica,calendar year of first exposure to silica | NA | NA | Yes | NM |
| Cowie et al.21 | 1153 (Exposed: 818, non-exposed: 335) | 49.2 | 1153 M | Gold miner | Occupation, age, date of diagnosis | Based on nodule profusion using the ILO standard guidelines, on entry to the study: Category 0 (n=335) Category 1 (n=418) Category 2 (n=355) Category 3 (n=45) | NA | Yes | RR: 2.8 (1.9 to 4.1) |
| Corbett et al.26 | 561 (Case: 381, Control: 180) | >35yr: 134, 35–44yr: 234, > 45yr: 293 | 561 M | Driller, miner, scraper winch, rock transport, dusty job | Occupation, age, duration of employment, HIV, grade of silicosis | Based on a six-point scale modified from the ILO classification system designed for use with standard-size chest radiographs: None: 340 (TB:198, control:142) Possible: 69 (TB:50, control:19) Probable: 48 (TB:40, control:8) Early: 43 (TB:37, control:6) High grade: 47 (TB:43, control:7) | NA | Yes | Multivariate OR categorized by the grade of silicosis none:1 (<0.001), possible: 1.6 (0.86–2.90), probable: 2.8 (1.24–6.46), early/high-grade: 4.9 (2.32–10.58) |
| Chang et al.25 | 707 | 53 | 702 M, 5 F | Caisson work | Occupation, age, gender, anti-tuberculosis treatment before the date of silicosis diagnosis, progressive massive fibrosis, size of opacities in CXR | NA | Simultaneous diagnosis of TB and silicosis: 71, <2 months after diagnosis of silicosis: 37, >2 months after diagnosis of silicosis: 143 | Yes | RR: 8.6 (NM) |
| Ross et al.23 | 370 (Exposed: 185, non-exposed: 185) | 43.95 | 370 M | Gold miner | Age, height, baseline lung function, years of employment, smoking, other respiratory diagnoses | NA | NA | Yes | OR: 1.86 (1.03-3.37) |
| Meel et al.22 | 271 | 51.6 | 300 M | Former mine worker (mostly worked in the mining industry for 11-15 years) | Age, duration of employment | X-ray images were classified according to International Labor Organization (ILO) standards and were entered in the Epi-info 6 program and then analyzedProbable: 26 Slight: 16 Marked: 19 Severe: 4 | <10 years: 23 11-20 years: 31 >21 years: 10 | Yes | OR: 5.08 (2.58-9.88) |
| Yarahmadi et al.27 | 1300 (Case: 871, Control: 429) | 40.77 | 725 M, 575 F | Casting, agriculture, undergroundmining, concrete manufacturing industry, constructionindustry, cement work, sand and gravel production, stonecarving, ferrosilicon company, mine, glass production,sandblasting, tunneling, brick making, construction work, stonemasonry, plaster work, road construction, stone milling, stone mining, asphalt company, limestone, granite stone, stone mine driver concrete production, installation, repair, and maintenance of railways, tiling, tessellation, tile and ceramic manufacturing industries, stone cutting, mosaic manufacturing, and pottery | Age, gender, the intensity of silica exposure, duration of employment, level of education, cigarette smoking | Degree of exposure to silica according to the classification of occupations based on level of exposure: No exposure: 740 (TB: 418, control: 322) Mild: 299 (TB: 235, control: 64) Moderate: 194 (TB: 159, control: 35) Severe: 67 (TB: 59, control: 8) | 15 years≥ silica exposure: 148 TB, 54 Non-TB; 15y< silica exposure: 305 TB, 53 Non-TB | Yes | OR: 4.08 (2.44-5.85) |
ILO: International Labor Office.
The diagnosis of silicosis was mainly based on chest X-ray findings; however, occupational history and physical examination were helpful. The most prevalent methods for tuberculosis diagnosis were sputum smear microscopy and culture (Table 2).
The follow-up duration reported in articles ranged from 3 to 20 years. The most common follow-up techniques were clinical evaluation, radiologic imaging, sputum examination, pulmonary function tests, and questionnaires (Table 2).
All studied populations were silicosis-confirmed patients, except one study,27 which stratified the population into silicosis and silica-exposed individuals without silicosis.
Disease severity classification was based on the level of exposure to silica27 and X-ray images were classified according to International Labor Organization (ILO) standards21,22,26 (Table 3).
All studies established the substantial effect of silicosis on tuberculosis development, with the estimated effect measures ranging from 1.86 to 8.623,25 (Table 3).
Several confounding factors were mentioned in our reviewed studies, including occupation, age, gender, height, HIV, smoking, duration of employment as an indicator for the time of silicosis or silica exposure, the severity of silicosis, co-existence of other pulmonary diseases, progressive massive fibrosis, baseline lung function, using anti-TB medications before silicosis diagnosis, and level of education (Table 3).
Since no data for confounding factors were available, we could not perform any stratification analysis.
One study evaluated tuberculosis risk factors in a silicosis cohort in Hong Kong and identified four TB risk factors as follows: no anti-tuberculosis treatment before the date of silicosis diagnosis (RR 4.51, 95%CI 2.46–8.24), progressive massive fibrosis (RR 3.78, 95%CI 2.25–6.36), small opacities exceeding 1.5 mm (RR 2.17, 95%CI 1.38–3.42), and caisson work (RR 1.56, 95%CI 1.01–2.41).25 Almost all samples had a history of mine work or dusty jobs. One study reported that 48.4% of their silicosis population had worked in the mines for 11 to 20 years.22
According to two studies22,27 older silicosis patients were mentioned as having a higher risk for TB. One study demonstrated that TB was most common in the age group of 36 to 45 years, and the age groups that were least susceptible to TB were those aged ≤35 years (2.6%) and 66+ years (7.4%).22
The frequency of TB increased along with the duration and intensity of silicosis/silica exposure. One case-control study showed that individuals with >15 years of moderate to severe silica exposure had the highest TB rate.27 Another study reported that the largest group of subjects with TB had a mining history of 11-15 years.22 Moreover, one study found that probable, early, and high-grade silicosis, as well as the period of employment, were significant risk factors for TB.26
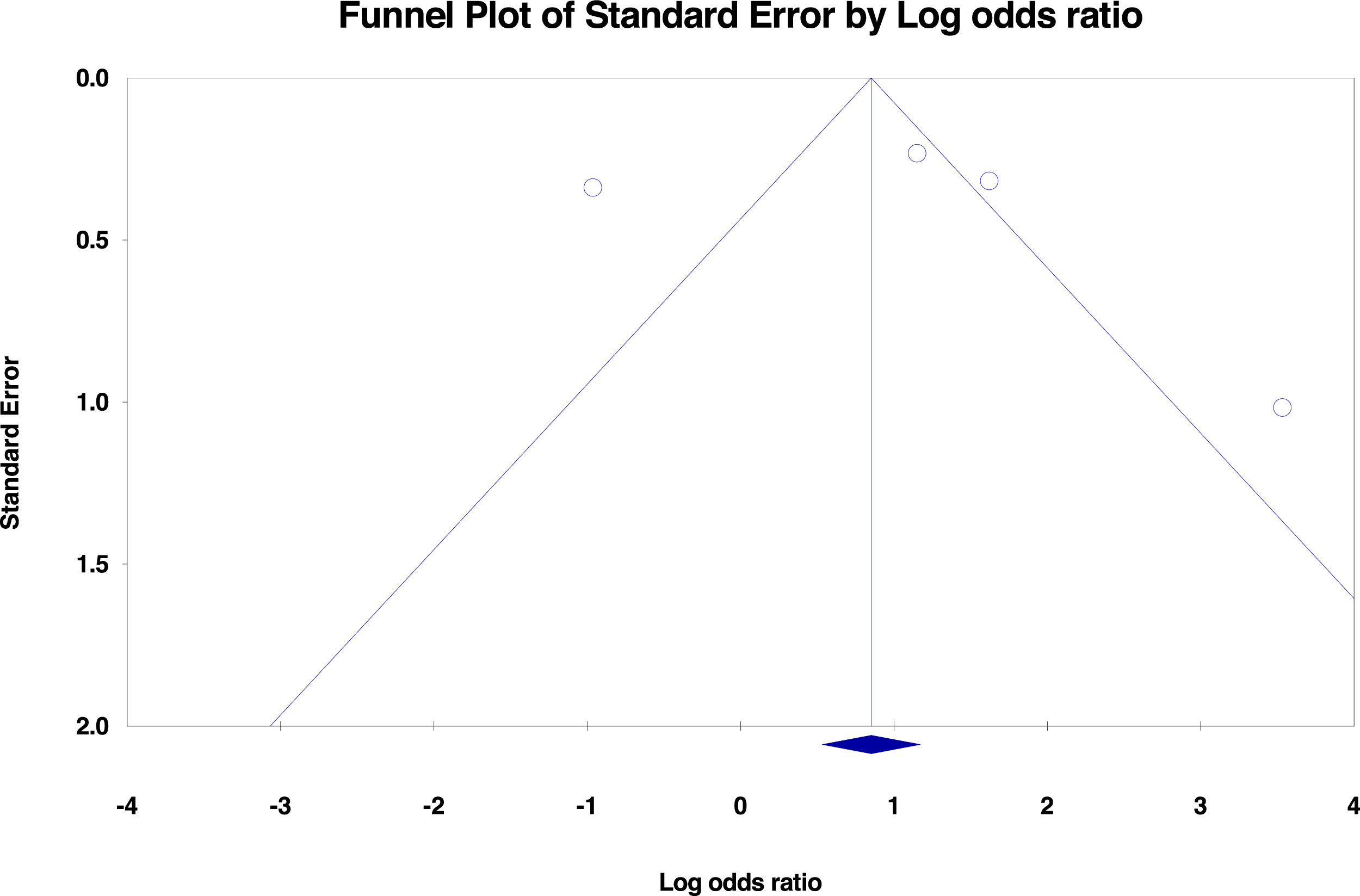
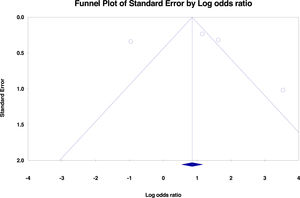
No significant publication bias was found in the selected studies (Begg's test P>0.05 and forest plot qualitative analysis) (Fig. 2). Pulmonary silicosis increased the risk of pulmonary tuberculosis, with a pooled risk ratio of 1.35 (95%CI 1.18-1.53) with an I 2 of 94.30% (Fig. 3). Considering the large outlier effect size of Westerholm et al.,24 we repeated the analysis without this study; however, the result did not significantly differ (RR 1.85, 95%CI 0.45-7.60, I 2: 94.41%) compared with the previous estimate.
DiscussionIn the present meta-analysis study, we found an increased risk of tuberculosis in silicosis patients and individuals exposed to silica dust.
The presence of silica particles in the lung and silicosis may facilitate the occurrence of tuberculosis infection and progress to disease, increasing the disease severity and favoring poor outcomes.29 According to some studies, exposure to silica affects the fibrotic tissue of the lung, and the chronicity and immunomodulation of silicosis cause susceptibility to Mycobacterium tuberculosis, leading to a significant incidence of tuberculosis in silica-exposed populations.3,29,30 Free silica can kill or damage alveolar macrophages, which play a major role in controlling tuberculosis bacilli.31,25 Silica exposure decreases cellular function and dendritic cell activation, leading to a nonspecific and impaired inflammatory response.3 Thus, susceptibility to bacterial infections, mainly Mycobacterium tuberculosis and other mycobacterial species, increase.3
Evidence supports a greater risk of HIV-infected silicosis individuals for TB, based on the existing defect in cellular immunity of HIV patients.31,25 The HIV epidemic impact on TB incidence in miners partly depends on the interaction between HIV and silicosis as risk factors for TB. The results from a study in South African miners demonstrate a high incidence of both silicosis and TB in HIV-positive miners, which are compatible with a multiplicative effect of silicosis and HIV infection.3 HIV's effect on the incidence of silico-tuberculosis was also discussed by studies included in our systematic review. Corbett et al. showed that HIV infection was a significant risk factor for both TB and nontuberculous pulmonary mycobacterial diseases in South African gold miners.26. They also suggested that as the South African HIV epidemic progresses, HIV infection may well make a significantly increasing addition to the already elevated rates of occupational mycobacterial diseases.26 This was supported by Cowie et al. in their epidemiologic study on South African gold miners.21 Another study found that HIV status at the time of TB diagnosis was not associated with loss of lung function. However, HIV status could not be analyzed as a confounder because it was tested only in the TB group.23
Occupation type is another major risk factor for developing silicosis and silico-tuberculosis.13 In the study by Farazi et al., the incidence of tuberculosis in silicosis patients was 28 to 39 times higher than that of the average population.8 Also, previous studies suggested that even silica exposure without the clinical diagnosis of silicosis may be a risk factor for tuberculosis.13,32,33 Workers may be at high risk for silica dust exposure in many occupations across a variety of industries. Calvert et al. estimated occupational silica exposure in several industries, using death certificates from the United States. They demonstrated that metal and coal mining, crude petroleum and natural gas extraction, non-metallic mining and quarrying, construction, cement, concrete, gypsum, and plaster products, pottery and related products, miscellaneous non-metallic mineral and stone products, iron and steel foundries, construction and material handling machines, and non-specific manufacturing industries were among the high-risk occupations regarding the silica exposure.34 Almost all of our studied population were employed in some of the mentioned industries (e.g., metal and coal mining, quarrying, tunneling, steel iron foundries, construction, etc.).
The majority of our reviewed studies made no mention of any specific duration for silicosis or silica exposure among patients; in some cases, the duration of service was presumed to be the estimate for time of the silica exposure; most of the former mine workers had work experience in diverse mining settings. Due to the dramatically varying dust-controlling efforts and systems from one mine to another, one cannot simply correlate years of service and the type and severity of lung disease. Thus, the measured dust levels in the mines and other related workplaces cannot offer reliable risk-assessment data for former workers whose lung diseases are due to lung tissue irritations occurring several years ago.
A cohort study on patients with silicosis in Hong Kong revealed that a multiplicative positive interaction might exist between silicosis and smoking effecting mortality rates of pulmonary tuberculosis.35 In another prospective cohort study on Chinese patients with silicosis, further support for a causal link between smoking and TB was found.36
However, we could not perform a subgroup analysis due to inadequate data on smoking status in the studies we included.
Various other risk factors have been demonstrated including older age, male sex, chronic obstructive pulmonary disease, to be migrated from endemic areas, the severity of the silicosis, exposure to toxic materials, intensity of the exposure, poor nutritional status, and low BMI.9,37,38 Further investigations with greater populations are required to elucidate the extent of each of the mentioned risk factors in TB development and progress.
Strengths and limitationsThe final result of our study is along with the Ehrlich et al. meta-analysis published in 2021 which demonstrated an increased risk of tuberculosis in silicosis patients.32 However, our study has some strengths and is different in some ways. We included studies focused purely on the relationship between silicosis and silica dust exposure and tuberculosis. Postmortem studies or studies in which the sample population had specific comorbidities (e.g., end-stage renal disease) were excluded to lessen the potential effect of the confounding factors. Also, we excluded the articles in which the full text was not found. Against this, we included two other studies that not previously included.22,23 Our calculated pooled risk ratio (1.35, 95%CI 1.18-1.53) was a little lower than Ehrlich et al. (RR 1.92, CI 95% 1.3-,2.73) but in line with its direction and statistical significance.
The major limitation of our study is its originality, as the data on the association between silicosis and tuberculosis has been known and ascertained for some time and also reported by international agencies NIOSH (2003), OSHA (2004) regarding the need for health surveillance for tuberculosis in silicosis patients and people at risk of silicosis. However, that association has resulted in very high rates of tuberculosis in countries with poor tuberculosis and silica exposure control and an update of data relating to scientific evidence can still have value.
Some other limitations of our study are associated with the studies we included and are as follows: First, there have not been any recent cohort and case-control studies on the topic and the most recent one was published in 2013. Further new investigations with a large number of populations are required to elucidate the issue. Second, the male gender was dominant in almost all of our included studies and only one study.27 had an equal male-to-female proportion. This could be because mine workers are mainly males but females can also be affected by working and living near workplaces.39 We suggest future studies to consider this issue. Third, several confounding factors were mentioned in our study which despite our inability to subgroup analysis, due to insufficient and inconsistent data, can inspire future research in this field.
ConclusionsSilicosis and silica dust exposure increase the risk of tuberculosis. Therefore, individuals with long-time silica exposure, like mine workers, should be routinely considered for both silicosis and tuberculosis screening programs. Dust control measures should be enforced and followed by focused tuberculosis control programs for individuals in high-risk jobs. Also, surveillance and reporting methods among these high-risk workers should be improved to monitor the effectiveness of preventive programs.
Authors contributionAll authors participated in the drafting and revision of the manuscript and the approval of the final version.
None.