Evidence is accumulating on the interaction between tuberculosis (TB) and COVID-19.
The aim of the present review is to report the available evidence on the interaction between these two infections. Differences and similarities of TB and COVID-19, their immunological features, diagnostics, epidemiological and clinical characteristics and public health implications are discussed. The key published documents and guidelines on the topic have been reviewed.
Based on the immunological mechanism involved, a shared dysregulation of immune responses in COVID-19 and TB has been found, suggesting a dual risk posed by co-infection worsening COVID-19 severity and favouring TB disease progression.
The available evidence on clinical aspects suggests that COVID-19 happens regardless of TB occurrence either before, during or after an active TB diagnosis. More evidence is required to determine if COVID-19 may reactivate or worsen active TB disease. The role of sequeale and the need for further rehabilitation must be further studied
Similarly, the potential role of drugs prescribed during the initial phase to treat COVID-19 and their interaction with anti-TB drugs require caution. Regarding risk of morbidity and mortality, several risk scores for COVID-19 and independent risk factors for TB have been identified: including, among others, age, poverty, malnutrition and co-morbidities (HIV co-infection, diabetes, etc.). Additional evidence is expected to be provided by the ongoing global TB/COVID-19 study.
The year 2020 will probably be remembered as the ‘COVID-19 (coronavirus disease) year’. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for this pandemic emerged in January/February, having originated from China in late 2019.1–3 Although COVID-19 continues to dominate both the scientific literature and the media, other communicable diseases including tuberculosis (TB) should not be neglected.4
Much has been written on the potential interactions between COVID-19 and tuberculosis (TB) following the World Health Organisation (WHO) declaration of COVID-19 as a Public Health Emergency of International Concern,5 initially based on assumptions, modelling6–8 and scientific evidence.9–13
The view of the WHO,7 and the specialized scientific press and newspapers14,15 is that an important consequence of the COVID-19 pandemic would be a worsening of the TB epidemic globally, for a variety of reasons, such as additional pressures on health systems by COVID-19 resulting in weakening of the National TB programmes16 and the potential biological effects of the interaction of the two infections, recalling the concept of ‘cursed duet’ which in the past was used for TB and HIV.17
The aim of the present review is to describe the available evidence on the interaction between COVID-19 and TB, starting from differences and similarities, proceeding to describe immunological features, diagnostic implications, epidemiological and clinical characteristics (including impact on mortality) and public health implications (impact on health services).
MethodsWe made a rapid and non-systematic search of the literature using the key-words ‘COVID-19′, ‘tuberculosis’, ‘immunology’, “diagnosis’, ‘prevention’, ‘treatment’, ‘infection control’, ‘workplace’ to identify a minimum set of references from an electronic database (PUBMED), existing guidelines on TB and COVID-19, airborne diseases, and grey literature. This review belongs to the Pulmonology TB series 2021.18
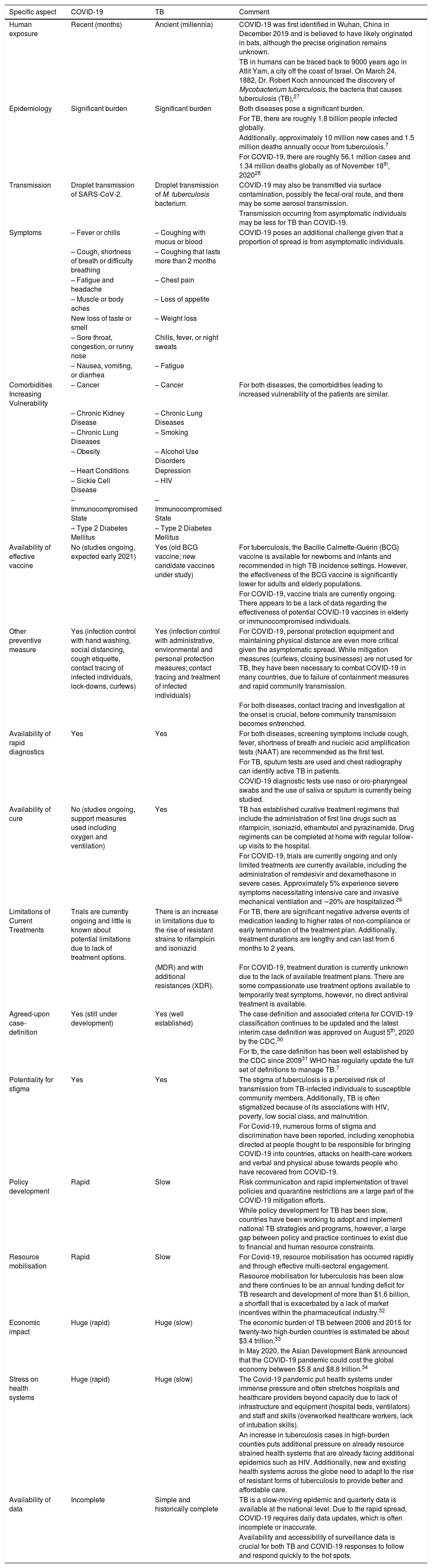
Differences and similarities between COVID-19 and TBRecent literature comparing,19–26 COVID-19 and TB are summarised with key similarities and differences in Table 1.
Differences and similarities between tuberculosis and COVID-19.
| Specific aspect | COVID-19 | TB | Comment |
|---|---|---|---|
| Human exposure | Recent (months) | Ancient (millennia) | COVID-19 was first identified in Wuhan, China in December 2019 and is believed to have likely originated in bats, although the precise origination remains unknown. |
| TB in humans can be traced back to 9000 years ago in Atlit Yam, a city off the coast of Israel. On March 24, 1882, Dr. Robert Koch announced the discovery of Mycobacterium tuberculosis, the bacteria that causes tuberculosis (TB),27 | |||
| Epidemiology | Significant burden | Significant burden | Both diseases pose a significant burden. |
| For TB, there are roughly 1.8 billion people infected globally. | |||
| Additionally, approximately 10 million new cases and 1.5 million deaths annually occur from tuberculosis.7 | |||
| For COVID-19, there are roughly 56.1 million cases and 1.34 million deaths globally as of November 18th, 202028 | |||
| Transmission | Droplet transmission of SARS-CoV-2. | Droplet transmission of M. tuberculosis bacterium. | COVID-19 may also be transmitted via surface contamination, possibly the fecal-oral route, and there may be some aerosol transmission. |
| Transmission occurring from asymptomatic individuals may be less for TB than COVID-19. | |||
| Symptoms | – Fever or chills | – Coughing with mucus or blood | COVID-19 poses an additional challenge given that a proportion of spread is from asymptomatic individuals. |
| – Cough, shortness of breath or difficulty breathing | – Coughing that lasts more than 2 months | ||
| – Fatigue and headache | – Chest pain | ||
| – Muscle or body aches | – Loss of appetite | ||
| New loss of taste or smell | – Weight loss | ||
| – Sore throat, congestion, or runny nose | Chills, fever, or night sweats | ||
| – Nausea, vomiting, or diarrhea | – Fatigue | ||
| Comorbidities Increasing Vulnerability | – Cancer | – Cancer | For both diseases, the comorbidities leading to increased vulnerability of the patients are similar. |
| – Chronic Kidney Disease | – Chronic Lung Diseases | ||
| – Chronic Lung Diseases | – Smoking | ||
| – Obesity | – Alcohol Use Disorders | ||
| – Heart Conditions | Depression | ||
| – Sickle Cell Disease | – HIV | ||
| – Immunocompromised State | – Immunocompromised State | ||
| – Type 2 Diabetes Mellitus | – Type 2 Diabetes Mellitus | ||
| Availability of effective vaccine | No (studies ongoing, expected early 2021) | Yes (old BCG vaccine; new candidate vaccines under study) | For tuberculosis, the Bacille Calmette-Guérin (BCG) vaccine is available for newborns and infants and recommended in high TB incidence settings. However, the effectiveness of the BCG vaccine is significantly lower for adults and elderly populations. |
| For COVID-19, vaccine trials are currently ongoing. There appears to be a lack of data regarding the effectiveness of potential COVID-19 vaccines in elderly or immunocompromised individuals. | |||
| Other preventive measure | Yes (infection control with hand washing, social distancing, cough etiquette, contact tracing of infected individuals, lock-downs, curfews) | Yes (infection control with administrative, environmental and personal protection measures; contact tracing and treatment of infected individuals) | For COVID-19, personal protection equipment and maintaining physical distance are even more critical given the asymptomatic spread. While mitigation measures (curfews, closing businesses) are not used for TB, they have been necessary to combat COVID-19 in many countries, due to failure of containment measures and rapid community transmission. |
| For both diseases, contact tracing and investigation at the onset is crucial, before community transmission becomes entrenched. | |||
| Availability of rapid diagnostics | Yes | Yes | For both diseases, screening symptoms include cough, fever, shortness of breath and nucleic acid amplification tests (NAAT) are recommended as the first test. |
| For TB, sputum tests are used and chest radiography can identify active TB in patients. | |||
| COVID-19 diagnostic tests use naso or oro-pharyngeal swabs and the use of saliva or sputum is currently being studied. | |||
| Availability of cure | No (studies ongoing, support measures used including oxygen and ventilation) | Yes | TB has established curative treatment regimens that include the administration of first line drugs such as rifampicin, isoniazid, ethambutol and pyrazinamide. Drug regiments can be completed at home with regular follow-up visits to the hospital. |
| For COVID-19, trials are currently ongoing and only limited treatments are currently available, including the administration of remdesivir and dexamethasone in severe cases. Approximately 5% experience severe symptoms necessitating intensive care and invasive mechanical ventilation and ∼20% are hospitalized.29 | |||
| Limitations of Current Treatments | Trials are currently ongoing and little is known about potential limitations due to lack of treatment options. | There is an increase in limitations due to the rise of resistant strains to rifampicin and isoniazid | For TB, there are significant negative adverse events of medication leading to higher rates of non-compliance or early termination of the treatment plan. Additionally, treatment durations are lengthy and can last from 6 months to 2 years. |
| (MDR) and with additional resistances (XDR). | For COVID-19, treatment duration is currently unknown due to the lack of available treatment plans. There are some compassionate use treatment options available to temporarily treat symptoms, however, no direct antiviral treatment is available. | ||
| Agreed-upon case-definition | Yes (still under development) | Yes (well established) | The case definition and associated criteria for COVID-19 classification continues to be updated and the latest interim case definition was approved on August 5th, 2020 by the CDC.30 |
| For tb, the case definition has been well established by the CDC since 200931 WHO has regularly update the full set of definitions to manage TB.7 | |||
| Potentiality for stigma | Yes | Yes | The stigma of tuberculosis is a perceived risk of transmission from TB-infected individuals to susceptible community members. Additionally, TB is often stigmatized because of its associations with HIV, poverty, low social class, and malnutrition. |
| For Covid-19, numerous forms of stigma and discrimination have been reported, including xenophobia directed at people thought to be responsible for bringing COVID-19 into countries, attacks on health-care workers and verbal and physical abuse towards people who have recovered from COVID-19. | |||
| Policy development | Rapid | Slow | Risk communication and rapid implementation of travel policies and quarantine restrictions are a large part of the COVID-19 mitigation efforts. |
| While policy development for TB has been slow, countries have been working to adopt and implement national TB strategies and programs, however, a large gap between policy and practice continues to exist due to financial and human resource constraints. | |||
| Resource mobilisation | Rapid | Slow | For Covid-19, resource mobilisation has occurred rapidly and through effective multi-sectoral engagement. |
| Resource mobilisation for tuberculosis has been slow and there continues to be an annual funding deficit for TB research and development of more than $1.6 billion, a shortfall that is exacerbated by a lack of market incentives within the pharmaceutical industry.32 | |||
| Economic impact | Huge (rapid) | Huge (slow) | The economic burden of TB between 2006 and 2015 for twenty-two high-burden countries is estimated be about $3.4 trillion.33 |
| In May 2020, the Asian Development Bank announced that the COVID‐19 pandemic could cost the global economy between $5.8 and $8.8 trillion.34 | |||
| Stress on health systems | Huge (rapid) | Huge (slow) | The Covid-19 pandemic put health systems under immense pressure and often stretches hospitals and healthcare providers beyond capacity due to lack of infrastructure and equipment (hospital beds, ventilators) and staff and skills (overworked healthcare workers, lack of intubation skills). |
| An increase in tuberculosis cases in high-burden counties puts additional pressure on already resource strained health systems that are already facing additional epidemics such as HIV. Additionally, new and existing health systems across the globe need to adapt to the rise of resistant forms of tuberculosis to provide better and affordable care. | |||
| Availability of data | Incomplete | Simple and historically complete | TB is a slow-moving epidemic and quarterly data is available at the national level. Due to the rapid spread, COVID-19 requires daily data updates, which is often incomplete or inaccurate. |
| Availability and accessibility of surveillance data is crucial for both TB and COVID-19 responses to follow and respond quickly to the hot spots. |
COVID-19: coronavirus disease; TB: tuberculosis; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; BCG: Bacille Calmette-Guérin; NAAT: nucleic acid amplification tests; MDR: multi-drug resistant; XDR: extensively drug-resistant; CDC: Centers for Disease Control and Prevention.
The main difference is that TB is curable, while definite evidence on effective anti-viral agents or other drugs for COVID-19 is still lacking.35,36
Research on new and effective vaccines is ongoing for both diseases: vaccination for COVID-19 has now started while for TB several candidates are under evaluation to replace the old BCG.37
Both COVID-19 and TB have the capacity to stress health systems, they are airborne transmissible diseases, can be diagnosed rapidly (although implementation of rapid testing is not yet available in all settings), they cause stigma and need public awareness and cooperation to allow prevention, diagnosis and treatment to be effective. Although surveillance is able to report on TB and viral diseases separately, in the vast majority of countries the information on COVID-19 is still incomplete and information on TB do not contain many clinical and immunological parameters, which would be useful to better understand the interaction between the two diseases. Moreover COVID-19 pandemic has led to a significant fall in TB notifications.9
In terms of funding, although health systems can be considered relatively underfunded even in resource rich countries (a debate is ongoing in these countries on the adequacy of prevention services and on the needed number of intensive care unit beds) human and economic resources for TB are historically sub-optimal at the global level, while resources have been rapidly mobilised against COVID-19 following the wave(s) of the emergency.19,20,38
A long story of prevention and control exists for TB, with the development of: a) national TB control programmes and b) prevention, diagnosis and treatment policies and guidelines in almost all countries of the world (although they are not always correctly implemented). On the COVID-19 side, the policy guidance is under continuous development, following the growing evidence available with the first and subsequent waves.
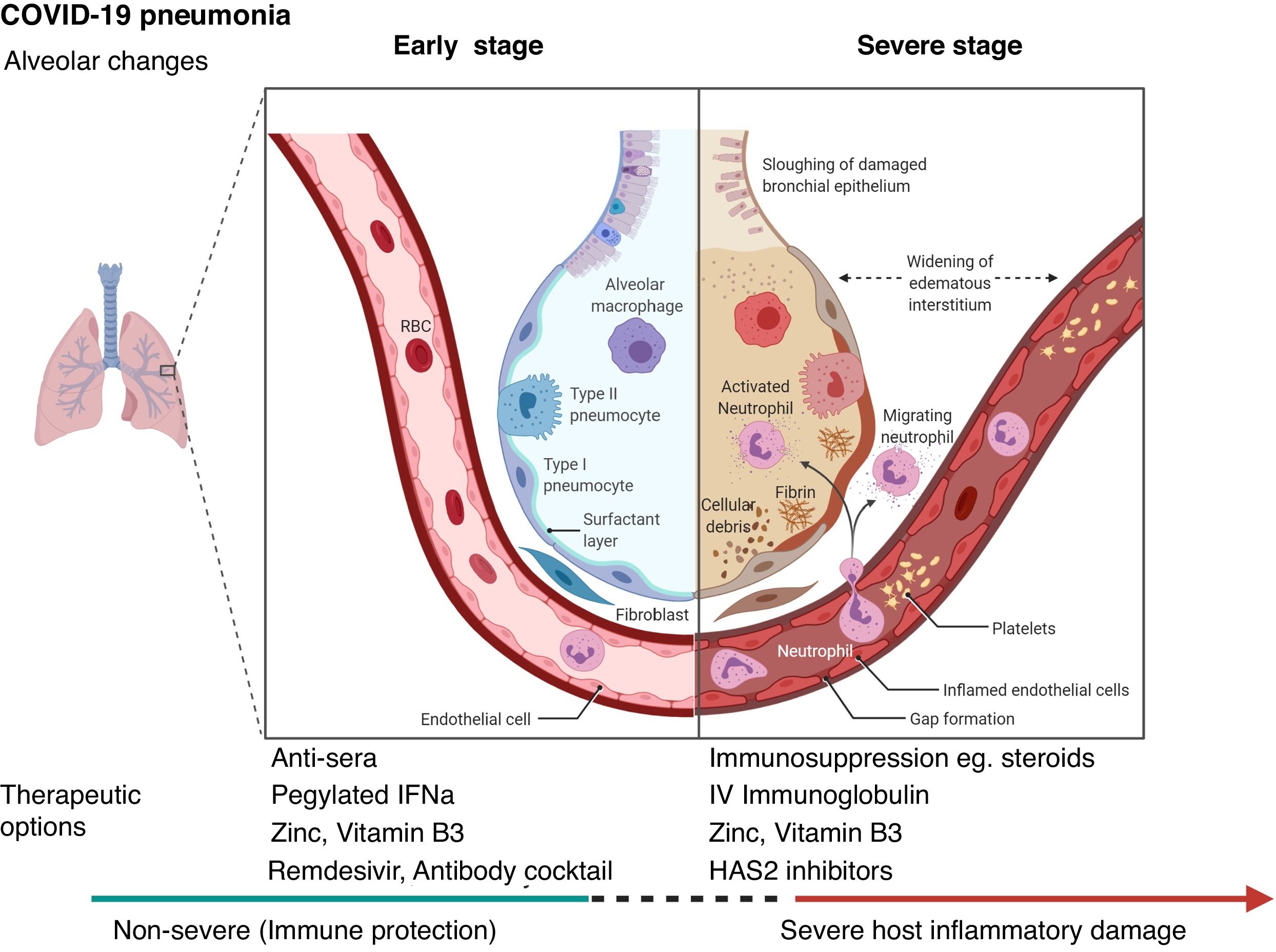
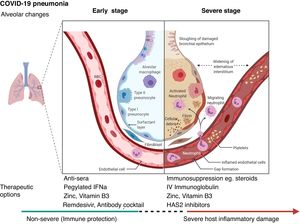
Biological interactionsCOVID-19 is a communicable disease caused by SARS-CoV-2, a member of the beta Coronaviridae family, which also includes SARS-CoV-1 (severe acute respiratory syndrome coronavirus 1) and MERS-CoV (Middle East respiratory syndrome coronavirus).39 The SARS-CoV-2 genome is up to 80% similar to SARS-CoV-1 and 50% similar to MERS-CoV.39,40 The coronavirus spike (S) glycoprotein, common to all these viruses, belongs to the class-I viral fusion proteins and upregulates and engages angiotensin-converting enzyme 2 (ACE2) as the entry receptor into humans.41,42 However, not all people exposed to SARS-CoV-2 are infected and not all infected patients develop severe respiratory illness.3 Accumulating evidence indicates that COVID-19 can be roughly divided into three stages: stage 1, an asymptomatic incubation period with or without detectable virus; stage 2, non-severe symptomatic period with the presence of virus; stage 3, severe respiratory symptomatic stage with high viral load43 and important immune response with subsequent deterioration of the lung damage, respiratory failure (that may require invasive-mechanical ventilation) and multi-organ dysfunction.44–47 (Fig. 1
It has been shown that a broad and coordinated SARS-CoV-2 antigen-specific adaptive immune responses (ADIMs) among CD4, CD8 and B cells are associated with lower COVID-19 disease severity, while absent or minimal adaptive immunity is associated with more severe COVID-19 disease. In particular SARS-CoV-2-specific CD4 + T cells are associated with protective immune responses.48 Significant redundancy or compensation may exist between the protective actions of neutralizing antibodies, SARS-CoV-2-specific CD4 T cells, and SARS-CoV-2-specific CD8 T cells.48
CD4 + T lymphocytes are rapidly activated to become pathogenic T helper (Th) 1 cells and generate granulocyte-macrophage colony stimulating factor (GM-CSF). The cytokine environment induces CD14+CD16+ monocytes with high expression of IL-6 and accelerates inflammation. Also, over-activation of T cells, manifested by the increase in Th17 and high cytotoxicity of CD8 + T cells in the peripheral blood of a patient with severe COVID-19, have been reported.49 Although the pathophysiology of SARS-CoV-2 is not yet fully understood, it seems there are similarities with that of SARS-CoV-1.50
Certain therapeutic interventions are under evaluation for the incubation and early stages of SARS-CoV-2 infection; these include convalescent plasma, pegylated IFNa (Interferon alpha), zinc, vitamin B3 and/or specific antivirals like remdesivir and Regeneron’s casirivimab/imdevimab antibody cocktail and bamlanivimab (Eli Lilly), some of which have already US Food and Drug Administration Emergency Authorization.51–53 However, the treatment with hydroxychloroquine and lopinvir/ritonavir has not been significantly associated with differences in hospital mortality.54,55
For patients with severe COVID-19, mostly immunosuppressive therapeutic options have been proposed, with dexamethasone being recommended for use and others currently being evaluated including HAS2 (Hyaluronan Synthase 2) inhibitors as well as activated MSCs (mesenchymal stromal /stem cells).44,56,57 (Fig. 1). Lung and tissue damage, which can occur with hypoxia even in TB,58 have also been described as sequelae to COVID-19 infection,59 as well as thrombosis and pulmonary emboli.47
Although viral respiratory infections and TB impair the host’s immune responses little evidence is available about co-infection of SARS-CoV-2 and Mycobacterium tuberculosis. TB status might play a role in the development of COVID-19 infection and exacerbation of the course of the disease for the co-infected population considering cases studied in China and India60 and the evidence provided by a study performed on a systematic transcriptomic evaluation of immune signatures associated with COVID-19 clinical severity and the spectrum of asymptomatic and symptomatic TB.17 In particular the results of this study performed on the transcriptomic evaluation of whole blood (WB), peripheral blood mononuclear cell (PBMC) and bronchoalveolar lavage fluid (BALF) signatures suggest that subclinical and active TB (ATB) increase the risk of severe COVID-19 disease, due to increased abundance of circulating myeloid subpopulations which are also found in the lungs of severe COVID-19 patients.17 The increased IFN production and the type I and III IFN responses signatures are significantly upregulated in severe disease in both COVID-1961 and TB62 and may lead to disease progression and severe/fatal outcomes. COVID-19 may therefore pose the biggest threat to ending the TB epidemic.6
Also, the use of immunosuppressive drugs in severe and critical COVID-19 patients, although done for a limited period of time, may result in increased likelihood of active TB caused by reactivation or new infection of M. tuberculosis63,64 even in post-pandemic times.
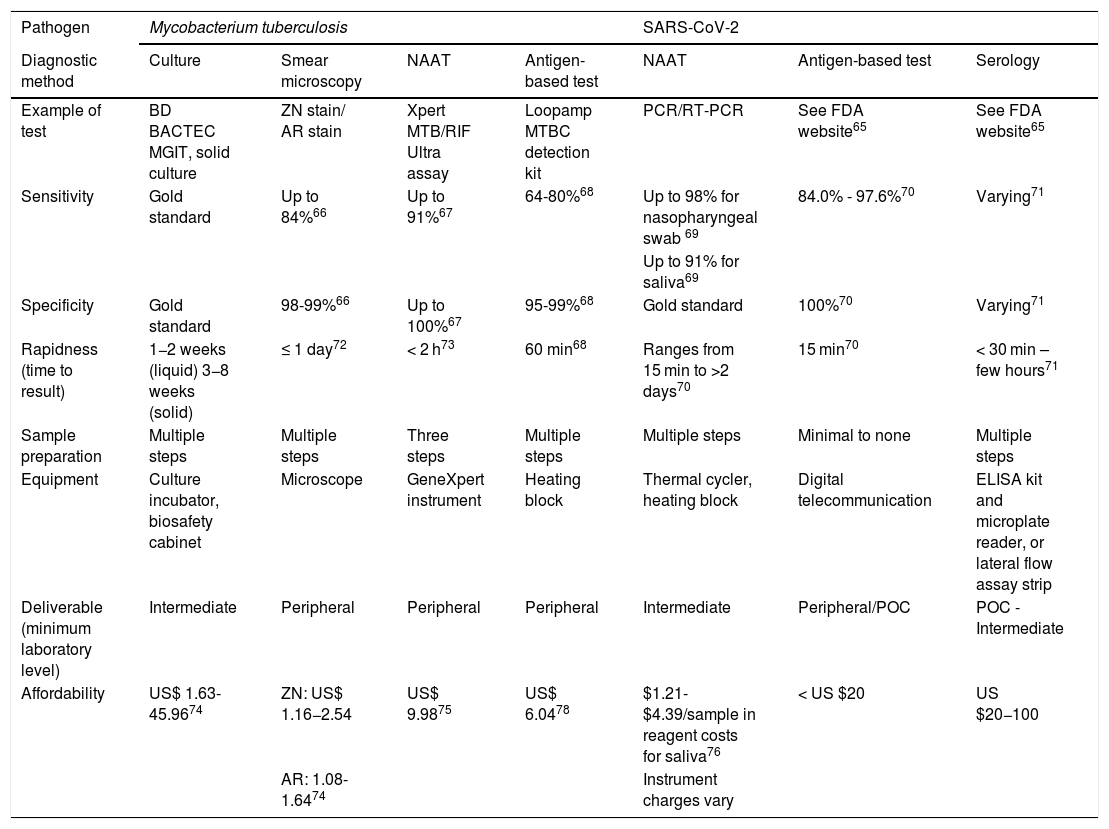
Diagnostic testsA range of diagnostic tests is available for both TB and COVID-19. For both pathogens, nucleic acid detection tests, and antigen-based tests are available while culture-based and smear methods apply to Mycobacterium tuberculosis and serology for SARS-CoV-2 (Table 2).
Diagnostic tests for M. tuberculosis and SARS-CoV-2.
| Pathogen | Mycobacterium tuberculosis | SARS-CoV-2 | |||||
|---|---|---|---|---|---|---|---|
| Diagnostic method | Culture | Smear microscopy | NAAT | Antigen-based test | NAAT | Antigen-based test | Serology |
| Example of test | BD BACTEC MGIT, solid culture | ZN stain/ AR stain | Xpert MTB/RIF Ultra assay | Loopamp MTBC detection kit | PCR/RT-PCR | See FDA website65 | See FDA website65 |
| Sensitivity | Gold standard | Up to 84%66 | Up to 91%67 | 64-80%68 | Up to 98% for nasopharyngeal swab 69 | 84.0% - 97.6%70 | Varying71 |
| Up to 91% for saliva69 | |||||||
| Specificity | Gold standard | 98-99%66 | Up to 100%67 | 95-99%68 | Gold standard | 100%70 | Varying71 |
| Rapidness (time to result) | 1−2 weeks (liquid) 3−8 weeks (solid) | ≤ 1 day72 | < 2 h73 | 60 min68 | Ranges from 15 min to >2 days70 | 15 min70 | < 30 min – few hours71 |
| Sample preparation | Multiple steps | Multiple steps | Three steps | Multiple steps | Multiple steps | Minimal to none | Multiple steps |
| Equipment | Culture incubator, biosafety cabinet | Microscope | GeneXpert instrument | Heating block | Thermal cycler, heating block | Digital telecommunication | ELISA kit and microplate reader, or lateral flow assay strip |
| Deliverable (minimum laboratory level) | Intermediate | Peripheral | Peripheral | Peripheral | Intermediate | Peripheral/POC | POC - Intermediate |
| Affordability | US$ 1.63-45.9674 | ZN: US$ 1.16−2.54 | US$ 9.9875 | US$ 6.0478 | $1.21-$4.39/sample in reagent costs for saliva76 | < US $20 | US $20−100 |
| AR: 1.08-1.6474 | Instrument charges vary | ||||||
AR, Auramine-rhodamine; NAAT, nucleic acid amplification test; PCR, polymerase chain reaction; POC, point-of-care; RT-PCR, real-time polymerase chain reaction; TB-LAMP, tuberculosis loop-mediated isothermal amplification;; ZN, Ziehl-Neelsen.
The WHO has described the ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable to end-users), relevant to both Mycobacterium tuberculosis and SARS-Cov-2, to identify the most appropriate diagnostic tests for most settings.77 However, a key limitation to all available tests, independent of the pathogen, is the inability to promptly declare if the pathogen is viable and infectious78 For SARS-CoV-2, the virus requires live eukaryotic cells to replicate, with a minimum turn-arou.nd-time of one week to determine viability. For Mycobacterium tuberculosis, culture results to determine viability require a minimum of 6 weeks. Even in this age of state-of-the-art technology, rapid information on the state of infectiousness of these two pathogens remains elusive. An interesting experimental approach to evaluate SARS-CoV2-specific response in the whole blood has been recently reported79,80. It describes that SARS-CoV2-specific response is detectable in the whole blood and is present during the acute phase79 as well as in the convalescents.80
Epidemiological and clinical presentation of COVID-19 with TB infectionIn a first meta-analysis of six studies from China on a few patients,81 the TB prevalence among COVID-19 patients ranged between 0.47 to 4.47%. The TB prevalence was higher among patients with severe COVID-19 than in non-severe ones (1.47%, 10/680 vs 0.59%, 10/1703; OR: 2.1; P = 0.24).
In a cohort from eight countries (Belgium, Brazil, France, Italy, Russia, Singapore, Spain and Switzerland)11 TB and COVID-19 were studied in 49 patients during the initial wave of the pandemic. TB was diagnosed before COVID-19 in 26 patients (53.0%), COVID-19 was diagnosed before TB in 14 ones (28.5%) while the diagnosis was concomitant in 9 patients (18.3%) (within the same week). Forty-two patients (85.7%) had active TB while 7 (14.3%) suffered post-cure TB sequelae. The authors concluded the following:
- 1)
COVID-19 can occur before, simultaneously or after the diagnosis of TB;
- 2)
The role of COVID-19 in boosting the development of active TB is yet to be established;
- 3)
The role of TB sequelae in COVID-19 evolution is also unclear, potentially being a risk factor for worsening outcomes;
- 4)
Further studies are needed to enable analysis of interactions and determinants of outcomes in patients with both diseases.
These findings have been confirmed by a similar study conducted in India.82
In an interesting clinical study conducted in a reference TB centre in Northern Italy, the Sondalo Hospital,13 detection of Sars-Cov2 was made in 20 patients (the majority being young migrants without co-morbidities) following nosocomial transmission. All patients received hydroxychloroquine and no antiviral drug was administered, with oxygen administered to 4 patients at admission and 3 during their hospital stay. A single elderly patient with advanced pulmonary TB and cachexia developed COVID-19 pneumonia and died 6 days after admission. The other 19 patients had a good clinical outcome. TB lesions at chest radiography did not worsen and only 4 patients had signs of newly developed pneumonia.
The data reported suggest the following:
- 1
Low rate of clinical and radiological deterioration may be associated to young age of most patients, low frequency of co-morbidities, good quality of healthcare service
- 2
Impact of COVID-19 on active TB appears to be manageable with proper care. Rigorous infection control and personal protection devices are crucial to prevent the risk of in-hospital transmission.83
In the meta-analysis mentioned above81 the risk of TB death was 1.4 times higher in COVID-19 patients. The findings of a recent study12 on 69 patients from 8 countries suggest the following:
- 1)
The case fatality rate in the overall cohort was 11.6% (8/69); 14.3% (7/49) in the 8 countries study11 and 5% (1/20, the single old patient with comorbidities) in the Sondalo Hospital study.13
- 2)
Mortality is likely to occur in elderly patients with co-morbidities;
- 3)
TB might not be a major determinant of mortality;
- 4)
Migrants experienced lower mortality, probably due to their younger age and lower number of co-morbidities. However, the authors commented that in patients with severe TB and/or with a disease caused by resistant strains of Mycobacterium tuberculosis, a higher mortality rate can be expected also in younger individuals.
In a recent modelling study based on data from the Philippines,84 the risk of death in TB patients co-infected with COVID-19 was 2.17 times higher than in non COVID-19 ones, with a shorter time-to-death. The risk of recovery in these patients was 25% lower than in non COVID-19 ones, with longer time-to-recovery.
A study from South Africa85 showed that while HIV-TB co-infection doubled the risk of death of TB patients compared to HIV-uninfected individuals, TB (both drug-susceptible and drug resistant) increased the hazard of COVID-19 death of 2.7. A lower increase (1.51) was reported in those with previous TB.
A global study on TB and COVID patients, coordinated by the Global Tuberculosis Network (GTN) and supported by the World Health Organization (WHO) is going on at present to improve the description of the interaction between the two diseases. As of October 13th 2020, 36 Countries/Regions joined the global study, with 132 Centres from 27 Countries/Regions having already provided data for 597 individual patients.86 The primary objective of the study is to describe the characteristics of patients with COVID-19 and TB (current or past), including diagnostic tests and prescribed therapies. The secondary objectives are: 1. To evaluate the logistic and organizational feasibility of a global repository for patients with COVID-19 and TB and 2) to describe the clinical outcomes (outcomes of COVID-19 disease, as well as interim and final treatment outcomes of TB patients).86
The GTN suggested several priority research questions to be answered with this global a study and others ones.
They include:
- 1
Does COVID-19 increase the risk of developing TB disease in individuals with TB infection?
- 2
What is the COVID-19 attributable risk on TB mortality?
- 3
What are the other determinants of mortality in TB−COVID-19 co-infected patients?
- 4
Is BCG vaccination protective for COVID-19?87
- 5
Do TB/COVID-19 co-infected patients require different management? (or in other words, which additional services are needed for these patients?)
- 6
What impact will COVID-19 have on TB services over the coming years, considering also the increasing effects of its second wave?
- 7
Are patients with post-TB sequelae a higher risk group for COVID? Do they suffer increased mortality or delayed cure? Do these patients require specific rehabilitation services?
According to recent studies, a high proportion of cases with post-TB treatment sequelae suffer from lung function impairment and poor Quality of Life (QoL). Preliminary data suggest that pulmonary rehabilitation is effective in patients with a previous history of TB.88–91
In addition, it has been well described that severe acute respiratory syndrome is the dominant finding of the acute phase of COVID-19 infection whilst functional impairment of patients surviving the COVID-19 acute phase has been poorly described. Recent studies suggested that early, post-hospitalization rehabilitative interventions should be recommended.92–94
Impact of the COVID-19 pandemic on TB servicesFew studies are available on the potential interaction of COVID-19 on the TB health services.9,15
The GTN global study9 evaluated patient attendances in TB health care units in 33 centres from 16 countries comparing the volume of TB-related healthcare activities in the first 4 months of the COVID-19 pandemic (January–April 2020) to the same period in 2019.9 The majority of the centres experienced reductions during their national lockdowns in the first 4 months of 2020, in TB-related hospital discharges, of newly diagnosed cases of active TB, of the total active TB outpatient visits, and of the new latent TB infections diagnosed (and related outpatient visits). In some centres, personnel initially attributed for TB service provision were re-prioritised to COVID-19. In addition, the decreased attendance to TB clinics was associated with patient fear of exposure to COVID-19 in the community or with disruptions of the services or struggle in accessing health services during lockdown. Conversely, national lockdowns favoured the increased use of telemedicine. In the TB centres surveyed in Australia, Russia, India, and the United Kingdom, telehealth service use increased.
A study carried out in Sierra Leone10 compared the number of patients assessed for presumptive TB and the number of those confirmed sputum smear positive in the first 4 months of 2020 with the number of cases reported in 2018 and 2019. The results show a significant drop of confirmed TB cases. Furthermore, the number of presumptive TB decreased in March/April 2020, with no treatment supervised nor cases of TB/COVID-19 coinfection or childhood TB detected in April 2020. The study shows the indirect impact of COVID-19 on TB care in a low-resource high TB-burden setting. The study suggests that Africa needs economic and technology support to strengthen its response to COVID-19 pandemic. Otherwise, all results achieved in recent years in the fight against TB may be lost.
Similar findings have been observed in Brazil,95 China,96 India,7,97 Iran,98 Nigeria99 and United States (migrants).100 A similar experience was reported on children in South Africa.101 In Korea, on the contrary, the impact of COVID-19 on the performances of the TB private sector project (PPM) was not observed.102 Repeat lockdowns of varying degrees are reported in countries which have recurrent COVID-19 waves, and severe consequences to TB services are therefore expected.26
ConclusionsCOVID-19 causes a spectrum of host immunological responses with asymptomatic individuals to severe cytokine-storm events that may be fatal. Immunosuppression including steroids used to treat COVID-19 may in future result in TB reactivation. Gold standard diagnostic tests for COVID-19 are PCR, and culture-based methods for TB, but an ideal point-of-care tests that can promptly inform if an individual is actively infectious with TB remains elusive.
COVID-19 can occur at any time during a patient’s TB journey, with worse outcomes for patients affected by active pulmonary TB disease. More evidence is needed to understand the potential of COVID-19 to favor reactivation of an exisiting TB infection. The aspecific signs and symptoms common to COVID-19 and TB may facilitate a rapid access to imaging services (chest radiography and/or computerized tomography) which may manifest signs of a pre-existing TB.
Avaliable data is insuffcient to understand the potential effect of COVID-19 on the TB patients’ treatment outcome,11,12,86 as in existing series the majority of these patients are still undergoing treatment.
Based on the information available so far, the main determinants of mortality for COVID-19 are age and co-morbidities, including HIV co-infection, poverty, diabetes and malnutrition, all of these also have an impact on TB mortality.
We need higher quality prospective studies to really answer the main research questions raised. In the meantime patients who had or have active TB especially people living with HIV co-infection should do their upmost to avoid getting COVID-19 and should be offered suitable vaccination when possible.
Declarations of interestNone.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
The Authors wish to thank Dr Martin van den Boom (World Health Organization Regional Office for Europe) for his useful comments on the manuscript.
The article is part of the scientific activities of the Global Tuberculosis Network (GTN and of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2017-2020- GBM/RC/LDA).