Whole lung lavage (WLL) is the gold standard technique for the treatment of Pulmonary Alveolar Proteinosis (PAP). In this paper we evaluated and discuss bilateral WLL, namely the procedure work-up and the therapeutic efficacy.
Six bilateral WLL performed through a careful adherence to a modified Royal Brompton Hospital (London) technique were carried out without major complications and were associated with clinical and functional improvement of the PAP patients submitted to this procedure.
As there are benefits in terms of time, patient comfort and cost effectiveness compared to unilateral WLL, associated with the efficacy and safety observed, bilateral WLL seems to be a suitable first choice for therapeutic lavage in PAP patients.
A lavagem pulmonar total (LPT) é a técnica de referência para o tratamento da Proteinose Alveolar Pulmonar (PAP). Neste documento avaliamos e discutimos a LPT bilateral, nomeadamente o procedimento levado a cabo e a eficácia terapêutica.
Foram executadas seis LPTs bilaterais, através da adesão cuidada a uma técnica modificada do Royal Brompton Hospital (Londres), executadas sem complicações de maior e foram associadas à melhoria clínica e funcional dos pacientes com PAP submetidos a este procedimento.
Como existem benefícios em termos de tempo, conforto para o paciente e eficiência em termos de custos, comparando com uma LPT unilateral, associada à eficácia e segurança observadas, a LPT bilateral parece ser uma primeira escolha adequada para uma lavagem terapêutica em pacientes com PAP.
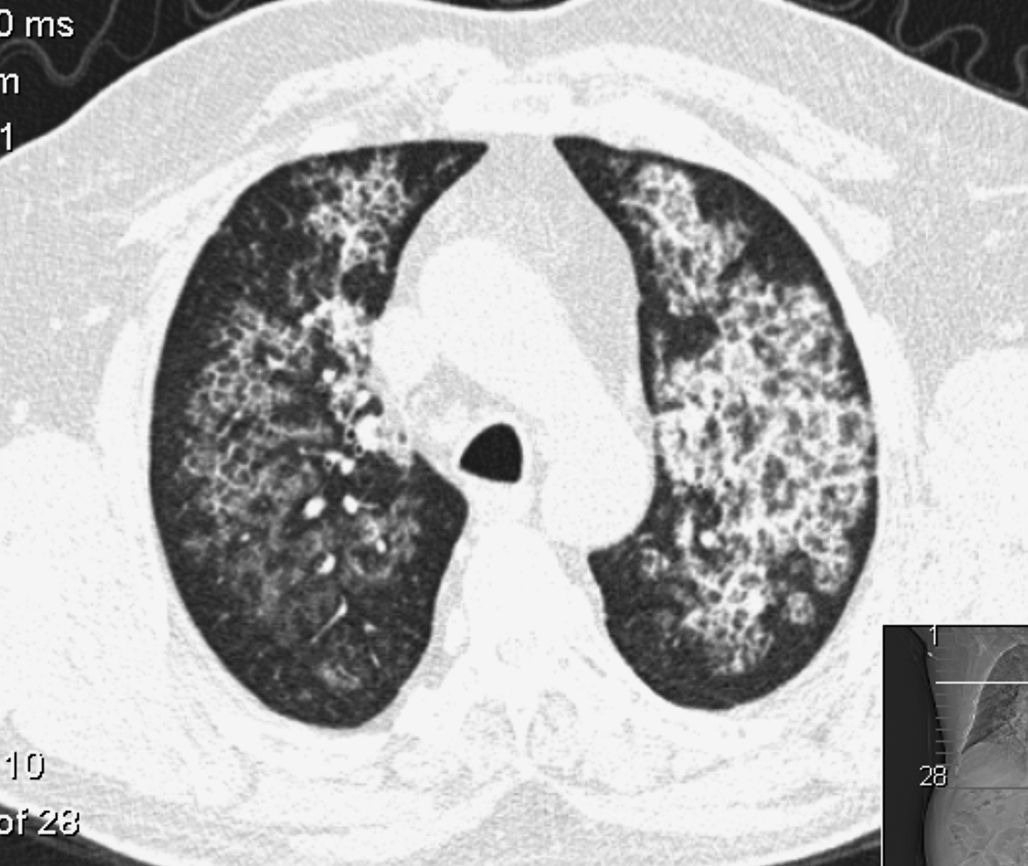
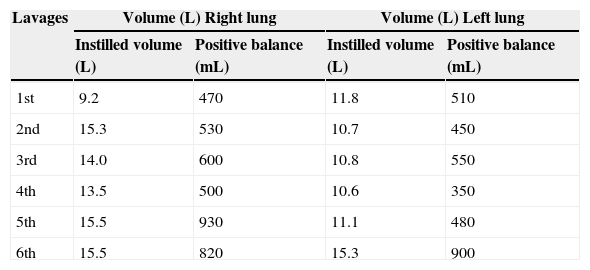
Pulmonary Alveolar Proteinosis (PAP) is a rare diffuse lung disease with three clinical forms, congenital, acquired (idiopathic) and secondary, characterized by an accumulation of large amounts of a phospholipoproteinaceous material in the alveoli due to a deficiency in granulocyte-macrophage colony-stimulating factor (GM-CSF) activity1–14 (Fig. 1). This is associated with an abnormal macrophage function and an impaired clearance of surfactant from the lungs. PAP has a prevalence of 3.7 cases per million, a male preponderance (4:1 male/female ratio) and 80% of the cases are reported during the third and fourth decade of life.6
Whole Lung Lavage (WLL), introduced in 1960s,3,4,7,10,15 is still the gold standard treatment.6 Unilateral WLL, with the lavage performed in each lung in different sessions separated by days/weeks, is the most frequent procedure. However, bilateral sequential WLL in the same treatment session is an attractive alternative, since it is significantly less time consuming, with a reduced amount of patient discomfort and is more cost effective.
Its efficacy has been attributed not only to the removal of lipoproteinaceous material from alveolar spaces, as well as the removal of anti GM-CSF antibodies, alveolar macrophages and type II epithelial cells. This therapeutic procedure is considered when a significant limitation in daily activities is reported by the patient and/or hypoxemia with a pO2<60mmHg, a P(A-a) O2 ≥40mmHg and a shunt fraction ≥10% is detected.12
In our Hospital, we performed the first WWL in 2010 and after five unilateral WLL, we moved on to a sequential bilateral WWL program.
The aim of this report is to describe the bilateral WLL technique and to discuss its safety and effectiveness.
MethodsIn this retrospective study, we collected demographic and clinical data from the medical reports of three adult patients. The standard technique applied was a modified version of the Royal Brompton Hospital (London) technique protocol.6,10
Throughout the procedure, electrocardiography, pulse oximetry (SatO2), invasive blood pressure, central venous pressure (CVP), urine output, capnography, tidal volumes bispectral index (BIS), and central temperature were continuously monitored and arterial blood gases (ABGs) were done hourly. A total intravenous anesthesia (TIVA) was performed to allow the depth of the anesthesia to be managed, independently of ventilatory variations inherent to the procedure. Curarization was maintained throughout the entire procedure. A left double lumen tube (DLT) was introduced, selecting the biggest size possible, to ensure lung isolation and promote ventilation and WLL efficacy. Its correct position was confirmed by fiberoptic bronchoscopy and cuff pressure insufflation measured in order to prevent contralateral leakage from the lavage fluid.11
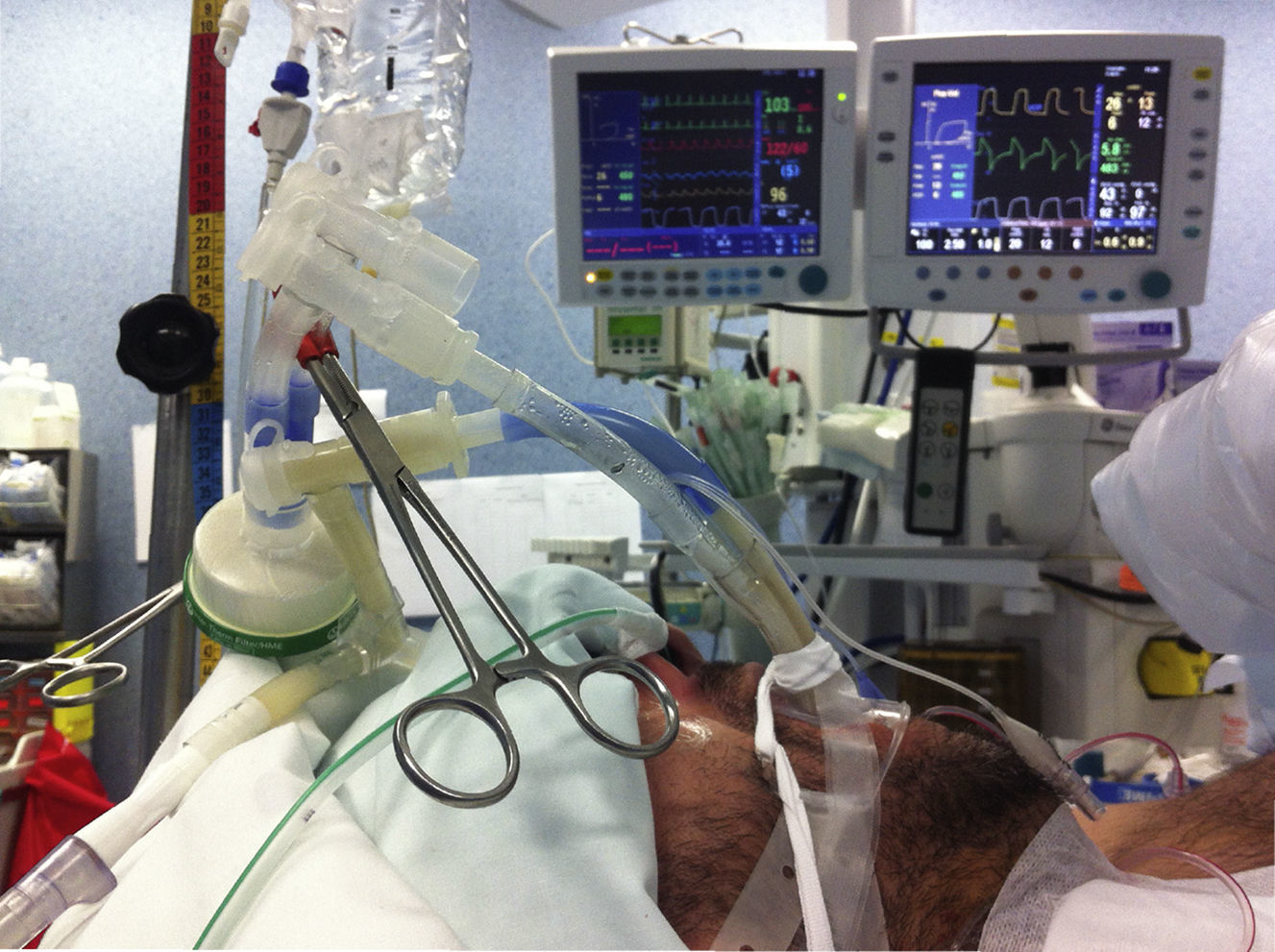
A pre-oxygenation with a FiO2 of 100% was carried out during 5min to ensure a correct alveolar denitrogenation and the occurrence of air bubbles, which could impair the removal of the lipoproteinaceous material from the alveoli. One-lung ventilation was started and lung isolation verified. Patients were ventilated by pressure-controlled ventilation, with pressure value under 30cmH2O. The volume of saline to be instilled was calculated by the preoperative measurements of functional residual capacity (FRC). The right lung volume was calculated by 3/5 of the FRC and the left lung by 2/5 FRC (in the first cycle a smaller volume was instilled). The saline should be instilled under gravitational effect from a height not exceeding 40cm above mid-axillar line, in order to prevent barotrauma and leakage to the ventilated lung.10 Although some reports describe a 30° lateral decubitus positioning in order to preserve the ratio of ventilation/perfusion of the dependent ventilated lung, this increases the probability of contralateral lung inundation, so we preferred a dorsal decubitus positioning, with a reverse trendelenburg as well as trendelenburg positioning to facilitate the gravitational instillation and removal of warm saline from the lungs.6,11 This positioning was adopted when a bilateral WLL was performed (Fig. 2).
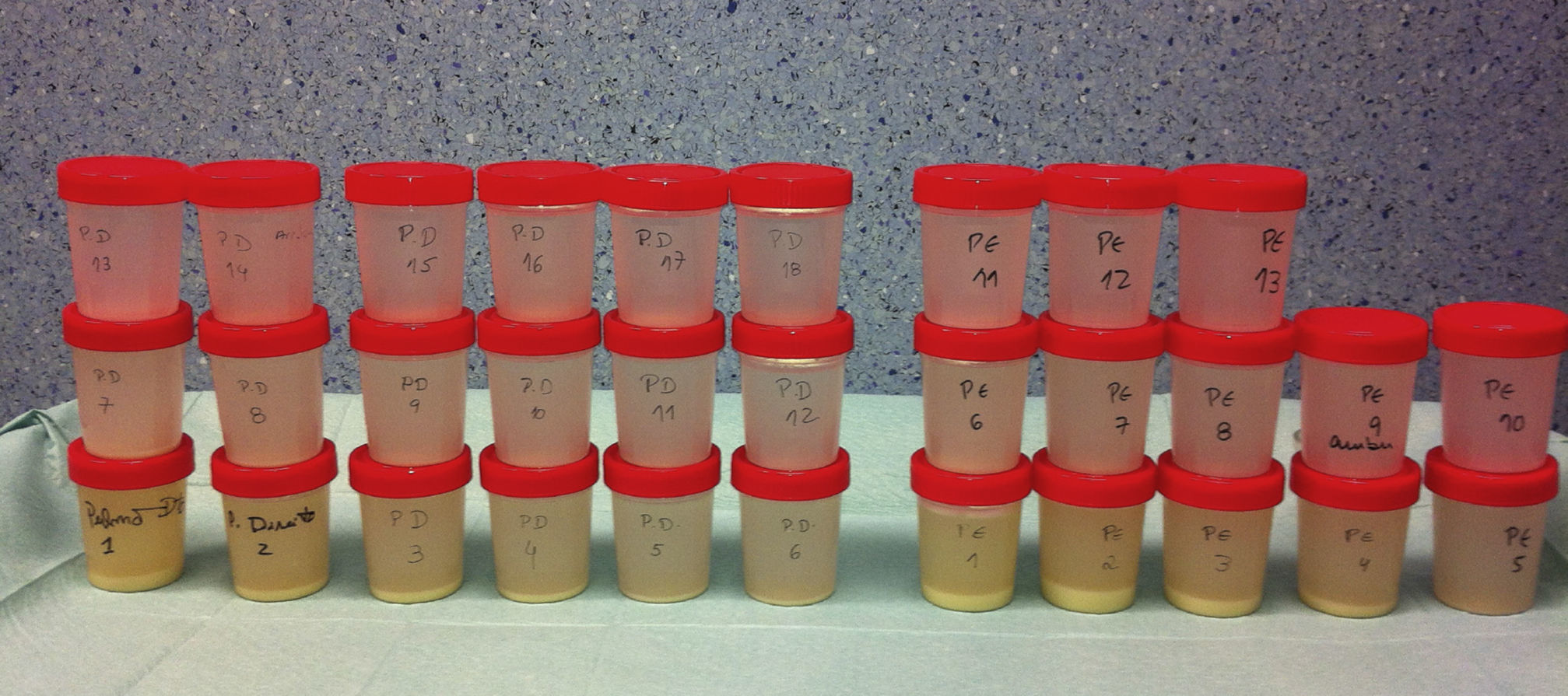
When the outflow, initially milky, became less dense, the drainage was interrupted at half of the volume, and several cycles of manual ventilation using a CPAP valve with 5–10mmHg pressure limit (the “Bingisser modification”) allowed for a manual percussion of the alveoli. This maneuver greatly enhances the lipoproteinaceous clearance.12,14 The lavage cycle was repeated until the lavage outflow became limpid (Fig. 3). Since the fluid lavage volume is high during procedure, there is a high risk of hypothermia. Body temperature conservation is crucial and should be maintained using heated lavage fluid at 37°C and a warming blanket.
After the first lung was successfully washed, it was carefully suctioned and its re-expansion was performed, initially by bilateral ventilation followed by unilateral ventilation. After 1h of ventilation, lung compliance and blood gas analysis data were verified. If the washed lung could secure the patient's gas exchange needs, then the contralateral WLL was initiated, repeating the procedures described above.8
At the end of the procedure the residual saline was aspirated through fiberoptic bronchoscopy and bilateral ventilation was resumed. The double lumen tube was then replaced by an endotracheal tube and the patient transferred to a recovery unit for overnight monitoring.
ResultsBilateral WWL was performed in three adult patients, 2 males and 1 female, mean age of 43,6 (range 39 – 47 years old) (Table 1) all of them with hypoxia at rest under 60mmHg.
Saline solution amount instilled and retained in bilateral WWL.
| Lavages | Volume (L) Right lung | Volume (L) Left lung | ||
|---|---|---|---|---|
| Instilled volume (L) | Positive balance (mL) | Instilled volume (L) | Positive balance (mL) | |
| 1st | 9.2 | 470 | 11.8 | 510 |
| 2nd | 15.3 | 530 | 10.7 | 450 |
| 3rd | 14.0 | 600 | 10.8 | 550 |
| 4th | 13.5 | 500 | 10.6 | 350 |
| 5th | 15.5 | 930 | 11.1 | 480 |
| 6th | 15.5 | 820 | 15.3 | 900 |
The first patient was male, 39 years old, smoker, baker, diagnosed with PAP one month before the first WLL, based on clinical, radiological and BAL features and the presence of serum GM-CSF antibodies. At disease presentation he had respiratory insufficiency associated with an extensive bilateral lung involvement observed in HRCT-scan and a bilateral WLL was performed with 9.2 and 11.8L of saline solution instilled into the right and left lung respectively. After a short period of clinical, functional and radiological improvement, the patient's condition worsened with the spread of the radiological lung opacities and respiratory insufficiency. This clinical deterioration coincided with his return to the workplace. In fact, bakery flour had been described as a potential trigger.16 A second bilateral WLL was performed three months later, and 15.3 and 10.7L of saline solution were then instilled. After this procedure and cessation of his previous work environment, the patient maintained clinical stability.
The second patient submitted to WLL was a female, 47 years old, farmer, non-smoker, no comorbidities, with the diagnosis of PAP one month before the first WLL based on clinical, radiological and BAL features and the presence of serum GM-CSF antibodies. The clinical presentation was very similar to the first patient's, with dyspnea on exertion and a dry cough associated with respiratory insufficiency and wide bilateral crazy paving pattern opacities in the HRCT scan. After the diagnosis, a WLL was performed with instillation of 14 and 10.8L of saline into the right and left lung respectively. After a brief initial clinical improvement, she was submitted to another WLL 1.5 months later, since she had become more symptomatic and with paO2<60mmHg. With this procedure, a total of 13.5 and 10.6L were instilled into the right and left lung respectively. Four months later a third WLL was performed because of clinical deterioration and at this time 15.5 and 11.1L were instilled into right and left lung respectively (Fig. 4). After this procedure, the patient achieved clinical, functional and radiological stability.
The third patient included was a male, 45 years old, ex-smoker, tire factory worker, with the diagnosis of PAP during a nocardia-induced cerebral abscess evaluation and treatment. As with the other two patients, the diagnosis was established by radiological and BAL typical features associated with serum GM-CSF antibodies. However, he had had a previous thoracic HRCT scan with bilateral crazy paving pattern two years earlier. One year after the diagnosis, he became more symptomatic, with dyspnea on exertion and respiratory insufficiency (paO2-57mmHg) and therefore was submitted to a WLL with instillation of 15.5 and 15.3 of saline into right and left lung respectively. After this procedure, he achieved significant clinical, functional and radiological improvement.
All six WLL procedures were carried out according to the established protocol and without any major complications.
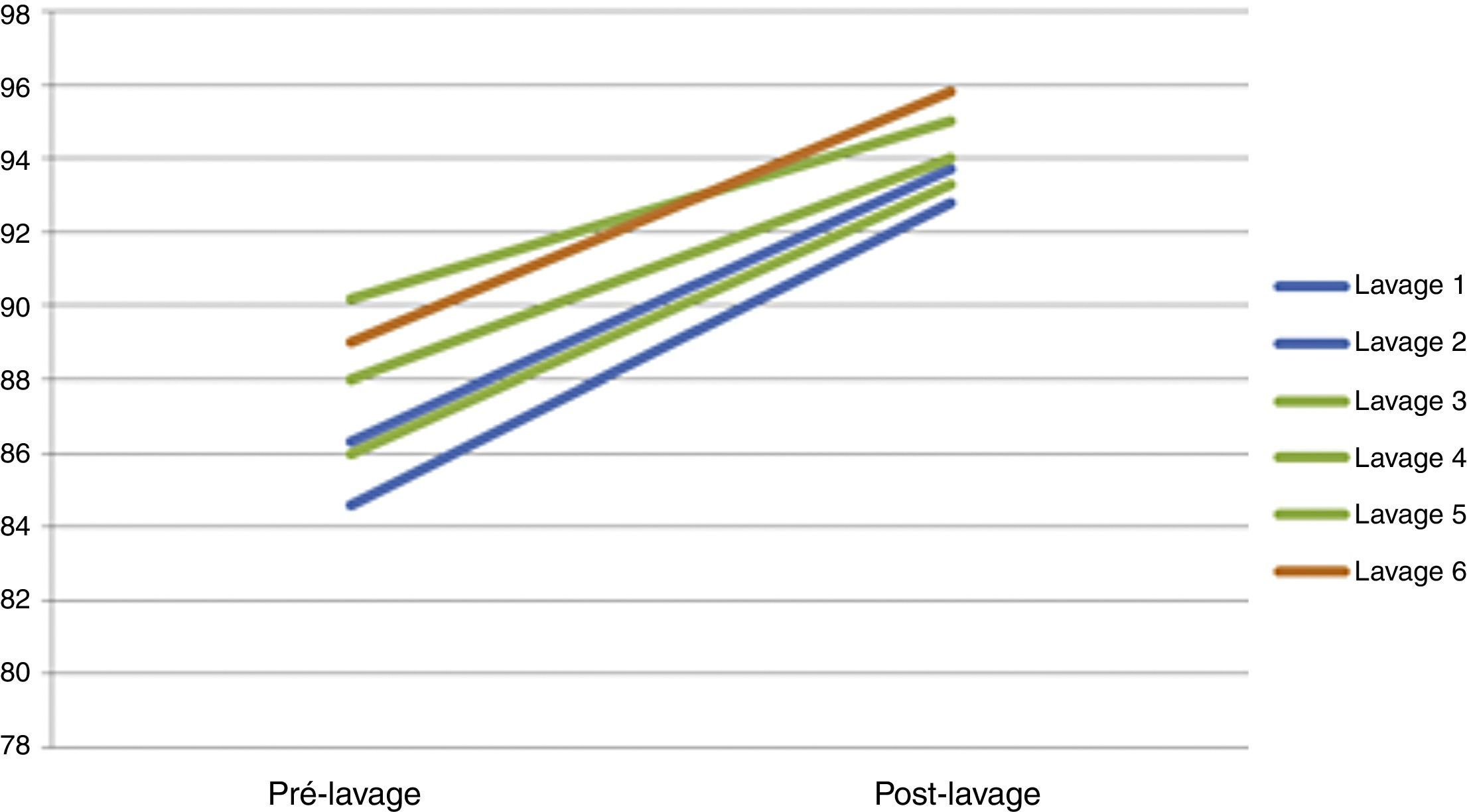
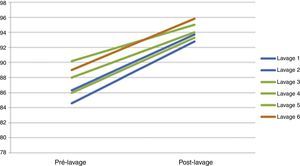
Hourly gasometrical monitoring focused on assuring correct oxygenation and ventilation. The patient gasometrical values were easily maintained throughout the procedure, with a pCO2 under 55mmHg and a SpO2 sustained above current patient's own values although they were in the supine position (Table 2) (Fig. 5). Indeed, in all bilateral WWL submitted patients we observed a progressive installation of metabolic acidosis during the procedure. This metabolic acidosis was reversed in the first hours postoperatively and no comorbidities were identified after that.
Blood gas analysis before and after bilateral WLL. lv: lavages.
| 1st pulmonar lavage | 2nd pulmonar lavage | 3rd pulmonar lavage | 4th pulmonar lavage | 5th pulmonar lavage | 6th pulmonar lavage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pré-lv | Post-lv | Pré-lv | Post-lv | Pré-lv | Post-lv | Pré-lv | Post-lv | Pré-lv | Post-lv | Pré-lv | Post-lv | |
| FiO2 (%) | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| pH | 7.44 | 7.48 | 7.4 | 7.45 | 7.44 | 7.49 | 7.46 | 7.48 | 7.47 | 7.51 | 7.41 | 7.46 |
| pO2 | 49.1 | 65.7 | 49.7 | 61.8 | 51.6 | 79.8 | 53 | 67 | 47 | 64.7 | 57.3 | 81.2 |
| pCO2 | 40.1 | 42 | 38.8 | 41.9 | 27.1 | 33.8 | 27.1 | 30 | 32.2 | 32 | 33 | 36.8 |
| HCO3 | 26.7 | 30.8 | 23.9 | 28.9 | 23.1 | 25.7 | 22 | 21.9 | 24.6 | 25 | 20.9 | 25.8 |
| SatO2 | 86.3 | 93.7 | 84.6 | 92.8 | 88 | 94 | 90.2 | 95 | 86 | 93.3 | 89 | 95.8 |
With the Bingisser maneuver we observed a transient difficulty in recovering volume lavage (contributing to a positive fluid balance) but an evident increase in lavage density.
In any case we observed an episode of contralateral lavage extravasation. In all procedures the body temperature was kept between 36 and 37°C. The mean procedure time was 8h (range 7h32–9h41).
Time to extubation varied according to the clinical and blood gas analysis evolution and all the patients were successfully extubated after 18h.
After these procedures the functional capacity and daily activities of all the patients were significantly improved, documented by clinical signs/symptoms as well as the respiratory function tests and gasometrical data.
DiscussionIn this short communication we described six bilateral sequential WLL procedures, performed in three patients with PAP, corroborating its clinical effectiveness and safety.
Despite its significant invasiveness, WLL remains the recommended treatment in PAP, and has been up till now the only therapeutic with established efficacy. In fact, when a patient with PAP presents with respiratory insufficiency and extensive imaging opacities at HRCT scan, this procedure is mandatory. WLL is an expensive and time consuming technique, demands the expertise of a multidisciplinary approach and this, along with the rarity of PAP explains why this procedure is only performed in a small number of centers. The choice of a bilateral WLL means better patient comfort, reductions in cost and time and keeping up clinical efficacy.
The three patients included could be considered typical cases of PAP, although there were some differences. Two of them had an acute presentation of the disease, needing WLL after the diagnosis while the other one was submitted to this procedure during the course of the disease. While this third patient had to be submitted to only one WLL, the other two had to have multiple WLL, to be precise to two and three procedures.
The crucial factor to be able to proceed to the second lung lavage is whether the first washed lung ventilation has recovered sufficient capacity to assure the patient's needs. For this reason, if there is no lateral predominance, we chose the larger lung, the right lung, to be washed first. To prevent barotrauma, maximum inspiratory pressure was limited to 30cmH2O. In all procedures, after 1h of ventilation, the pulmonary compliance and gasometrical values in one-lung ventilation of washed lung were compatible with the beginning of the lavage of the second lung.
The choice of pressure-controlled ventilation allowed us to continuously monitor changes in lung compliance during the lavage and prevented possible tube dislocation or any other interference. Moreover, in the same way, it permitted the washed lung compliance recovery to be observed.
The supine position of the patient did not hinder oxygenation, and so we did not have to resort to the lateral decubitus position with ventilation of the dependent lung, positioning that is less stable and entails greater risks in a lengthy procedure (average 8h).
We chose the Bingisser maneuver percussion due to the fact that the pressure exerted on the airways is measured, unlike in kinesiotherapy maneuvers, where that quantification is impossible, thus reducing the risk of excessive percussion pressure, avoiding and preventing a possible contralateral inundation.8
In an attempt to reverse the metabolic acidosis observed during the procedures, the maintenance serum used in the perioperative period – sodium chloride 0.9% – was replaced by ringer's lactate in the third bilateral procedure and thereafter, with only modest improvements. In the literature there is no reference to the use of this solute as lavage fluid for lung, so sodium chloride remains in use.
In conclusion, considering the clinical efficacy of the bilateral WLL, its advantages in saving time and costs and reducing patient discomfort make this technique a suitable first choice in therapeutic lavage in PAP patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.