Although catamenial hemothorax compared to pneumothorax is a rarer clinical presentation of thoracic endometriosis syndrome (TES), it is more commonly associated with diaphragmatic fenestrations. These openings may serve as entry portals for peritoneal fluid to access into the pleural space thereby perpetuating recurrent pleural effusion even after prior surgical pleurodesis. We report our experience with two patients with recurrent right catamenial hemothorax after previous interventions that were subsequently treated by talc pleurodesis and goretex diaphragmatic patch, and who have had no further recurrence at a mean follow up of 15 months.
We therefore recommend that diaphragmatic patch should be considered as an adjunct to talc pleurodesis in patients with recurrent catamenial hemothorax when either multiple diaphragmatic fenestrations are seen at surgery or if there is concomitant bloody peritoneal fluid which could potentially lead to recurrence. The patch by sealing any occult pores and possible future fenestrations appear to decrease recurrent pleural effusion at an intermediate term follow up.
Embora o hemotórax catamenial comparado com o pneumotórax seja uma apresentação clínica mais rara de síndrome de endometriose torácica (TES), está mais associado a fenestrações diafragmáticas. Estas aberturas podem atuar como portais de entrada para o acesso ao fluido peritoneal na cavidade pleural, perpetuando assim o derrame pleural recorrente mesmo após uma pleurodese cirúrgica prévia. Registamos a nossa experiência em dois pacientes com hemotórax catamenial recorrente do lado direito após outras intervenções, que foram posteriormente tratados com pleurodese com talco e penso diafragmático em gore-tex, e que não apresentaram nenhuma outra recorrência durante um acompanhamento de 15 meses.
Recomendamos, então, que o penso diafragmático seja considerado um auxiliar à pleurodese com talco em pacientes com hemotórax catamenial recorrente, tanto quando são vistas várias fenestrações diafragmáticas na cirurgia, como quando há fluido peritoneal hemorrágico concomitante, que poderá conduzir a uma recorrência. O penso, ao selar qualquer poro oculto e possíveis fenestrações futuras, parece diminuir o derrame pleural recorrente num seguimento a médio prazo.
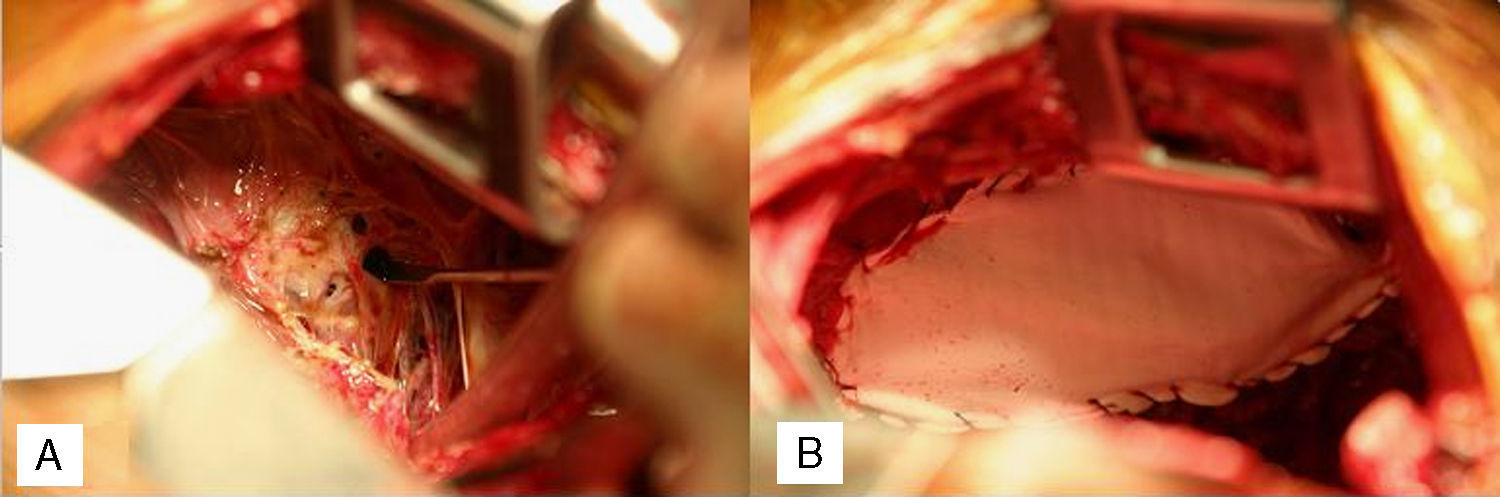
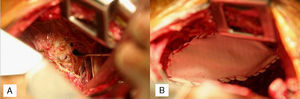
A 35 year nulliparous African American female who had had her last menstrual period a week previously, presented with dyspnea, right chest pain, abdominal pain and distension with onset of menses. Past medical history were primary infertility, thoracentesis for right hemothorax, video assisted thoracoscopy (VATS) for recurrent hemothorax, diagnostic laparoscopy and left ureteric stent for hydronephrosis, all 5 years earlier. She had had thoracotomy, decortication for recurrent hemothorax 5 months after the VATS. The previous diagnostic laparoscopy had revealed dense pelvic adhesions involving the uterus, ovaries and colon with retroperitoneal fibrosis causing left ureteric stricture and hydronephrosis. The patient had declined a hysterectomy at the time and was therefore treated medically with Lupron (Leuprolide) for 6 months followed by oral contraceptives. Paracentesis was repeated twice over the following 3 years for recurrent ascites. Admission workup at presentation included a chest X-ray, chest and abdominal CT which showed eventration of the right diaphragm, loculated pleural effusion, ascites and right ovarian cyst. She then underwent a redo-thoracotomy which revealed dense pleural adhesions, multiloculated hemothorax, with old bloody fluid seen entering from the abdomen through multiple diaphragmatic fenestrations with the largest measuring 2.5cm. These were closed individually with 3/0 prolene sutures, followed by a Gore dualmesh patch (Gore & Associates, Newark, Delaware, USA) covering of the diaphragm with 3/0 prolene, followed by talc pleurodesis. (Fig. 1A and B) Biopsies, cytology and cultures were all negative. Postoperative course was uneventful and she has had no recurrence at 18 months.
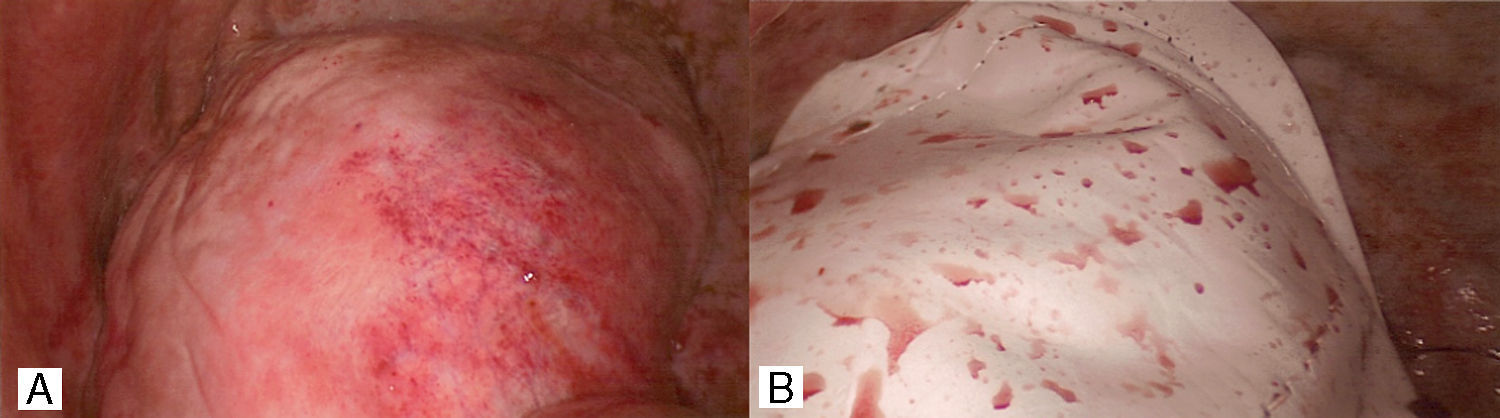
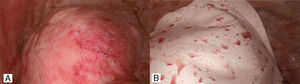
Second patientA 31 year old African American female presented with history of weakness, abdominal bloating, distension and dyspnea at rest which had progressively gotten worse with onset of her menses 2 days earlier. She admitted to similar symptoms with each menstruation over the last 6 months. She also had history of dyspareunia and secondary infertility with inability to conceive after her first childbirth 11 years earlier. Past surgical history was significant for Appendectomy and Myomectomy 4 years earlier. Abdominal and pelvic ultrasound showed large pelvic and abdominal ascites. Chest X-ray and Chest CT scan showed opacification of the right hemothorax for which patient had initial thoracentesis with drainage of over 2l of bloody effusion. Following reaccumulation of the hemothorax within a week, patient then underwent a right VATS pleural, diaphragmatic biopsies, goretex diaphragmatic patch and talc pleurodesis (Fig. 2). The diaphragm looked erythematous and inflamed with dark brown reddish nodules. The biopsies were however all negative for endometriosis. Her post operative course was uneventful and patient has had no recurrence of hemothorax at 12 months follow up.
DiscussionEndometriosis is estimated to affect 10-15% of women in their reproductive years.
Extrapelvic manifestation as thoracic endometriosis syndrome (TES) is relatively rare and in the largest series to date, Joseph and associates1 in a retrospective review of all published cases in the English literature comprising 110 patients, revealed that 73% of the patients had pneumothorax, 14% hemothorax, 7% hemoptysis and 6% lung nodules. Similarly in a review of 43 patients with TES by Hibbard et al.2 pneumothorax was the most frequent presentation in 58%, followed by Hemothorax 19%, Hemoptysis 16% and 7% asymptomatic. The etiology of TES is multifactorial with three proposed theories: lymphatic or hematogenous embolization from the uterus; coelomic metaplasia and retrograde menstruation with transdiaphragmatic migration via congenital or acquired diaphragmatic defects. The diaphragmatic fenestrations seen in the first patient were probably acquired from hormonal induced cyclical necrosis of diaphragmatic endometrial implants as proposed by Alifano et al.3 In the second patient although there were no visible fenestrations/holes seen at surgery, the rapid reaccumulation of right hemothorax after the initial drainage by thoracentesis and the subsequent failure to reaccumulate after the diaphragmatic patch support the possibility of occult diaphragmatic holes as the likely etiology in this patient as well. Histological evidence for TES is infrequent with only 37% and 52% of patients in the series by Hibbard et al.2 and Korom et al.4 respectively had positive histology. Treatment of TES is controversial, with advocates for both medical and surgical treatments depending on individual circumstances and desire for children. Hormonal therapy is used to suppress the cyclical activities of endometrial implants with amelioration of symptoms, although often associated with high recurrence on discontinuation of the drug. For instance, Joseph and Sahn1 report a 62% recurrence rate for hormonal therapy compared to 25% for surgical pleurodesis at one year with catamenial pneumothorax (CPTX) and in patients with catamenial hemothorax (CHT), 60% initially treated with hormone therapy also required surgery for recurrence.
Because definitive surgical treatment to remove the primary endometrial source by hysterectomy and bilateral salpingo-oophorectomy is often resisted by patients, who are usually young and still hoping to have children, surgical intervention is therefore usually directed at the local site of manifestation in the chest. Standard surgical treatment with pleurodesis alone for CPTX and CHT while superior to hormonal therapy still carries a significant incidence of recurrence especially with CHT where there is a higher incidence of diaphragmatic holes. To address this problem, Bagan et al.5 had reported 3 patients with recurrent CPTX and diaphragmatic defects repaired with polygalactin mesh with no recurrence at 35 months. They suggested that the mesh would cover any residual occult defects and also induce fibrotic adhesions to the lung. Since diaphragmatic fenestrations are more prevalent in CHT than CPTX cases with 71% versus 26% rates respectively in the series by Joseph and Sahn,1 it is logical to expect that they would be even more effective in reducing recurrence with CHT. Both our two patients treated with diaphragmatic patch covering have had no further recurrence at 18 and 12 months respectively following surgery. We therefore also would recommend a consideration of the adjunctive use of diaphragmatic patch with talc pleurodesis in recurrent CHT in patients found to have multiple fenestrations at surgery and those with bloody ascites even if there are no visible diaphragmatic pores as a means of reducing risk of further recurrence.
Conflicts of interestThe authors have no conflicts of interest to declare.