The authors present two case reports of necrotizing pulmonary aspergillosis. This disease is part of a spectrum of clinical conditions caused by the inhalation of Aspergillus spores. The necrotizing pulmonary aspergillosis (NPA) corresponds to an indolent, destructive process of the lung due to invasion by Aspergillus species, usually A. fumigatus. The diagnosis is confirmed by a histological demonstration of tissue invasion by Aspergillus species and its growth on culture. Due to the difficulty in confirming the diagnosis, the following diagnosis criteria were established and when combined are highly indicative of NPA: characteristic clinical and radiological findings, elevation of inflammatory markers and either serological results positive for Aspergillus or the isolation of Aspergillus from respiratory samples. Active tuberculosis, non tuberculosis mycobacteriosis, cavitary histoplasmosis and coccidioidomycosis should be excluded. It is necessary to raise the level of suspicion and perform the adequate diagnostic tests in order to start therapy and avoiding disease progression.
Os autores apresentam dois casos clínicos de aspergilose pulmonar necrotizante. Esta patologia faz parte de um espectro de condições clínicas provocadas pela inalação de esporos do fungo Aspergillus. A aspergilose pulmonar necrotizante (APN) corresponde a um processo indolente de destruição do pulmão pelo Aspergillus, geralmente A. fumigatus. O diagnóstico definitivo faz-se através da demonstração histológica de invasão tecidual pelo Aspergillus e do seu crescimento em cultura2. Pela dificuldade em obter um diagnóstico definitivo foram estabelecidos os seguintes critérios de diagnóstico que, quando reunidos, são fortemente indicativos de APN: aspectos clínicos e radiológicos consistentes com o diagnóstico, elevação dos parâmetros inflamatórios (PCR, VS) e marcadores serológicos positivos para Aspergillus ou isolamento de Aspergillus em amostras do aparelho respiratório. Deve ser feita a exclusão de tuberculose activa, micobacterioses não tuberculosas, histoplasmose cavitária e coccidiomicose. É necessário elevar o grau de suspeição desta patologia e realizar os exames de diagnóstico indicados de forma a iniciar terapêutica atempadamente.
Pulmonary aspergillosis is the name given to a variety of lung diseases caused by the Aspergillus fungus. 1 About 200 species of this fungus are already known, however, only a few are known to be pathogenic for humans.1 The most frequent ones are: A. fumigatus (75–85 %), A. flavus (5–10 %), A. niger (1.5-3 %) and A. terreus (2–3 %). 1 The Aspergillus is widely spread, mainly in rotting organic waste, dust, food and ventilation systems. 1,2 Transmission is made by inhalation of airborne spores which then get deposited in the respiratory tract.1,2
Clinical manifestations due to infection depend on the virulence of the fungus, intensity of exposure, patient's immunological satus and presence of previous lung diseases.1 Usually, in healthy individuals fungal inhalation does not cause disease.
Clinical case No. 1A Caucasian 47 year-old male patient, naval welder, former smoker (50 pack years) with significant drinking habits, was admitted with a 1-month history of cough, mucopurulent sputum, non-quantified evening fever, asthenia, anorexia, and weight loss of about 6 kg. Regarding past medical history, the following were relevant: pulmonary tuberculosis at 31 years of age, and reactivation at 43, followed by upper left lobectomy due to pulmonary nodes (tuberculomas).
Upon admission, the patient was emaciated, hemodynamically stable, with tympanic temperature of 37.7 °C, eupneic, with peripheral oxygen saturation (SpO2) of 98 % (FiO2 21 %) and with no alterations on pulmonary auscultation.
Laboratory results showed: Hb, 11.8 g/dL (normocytic-normochromic anaemia); leukocytes, 21.4 × 109/L (neutrophils, 69 %; linphocytes, 15 %); C-reactive protein (CRP), 5.8 mg/dL; erythrocyte sedimentation rate (ESR), 102 mm during the first hour; HIV 1 and 2 negative.
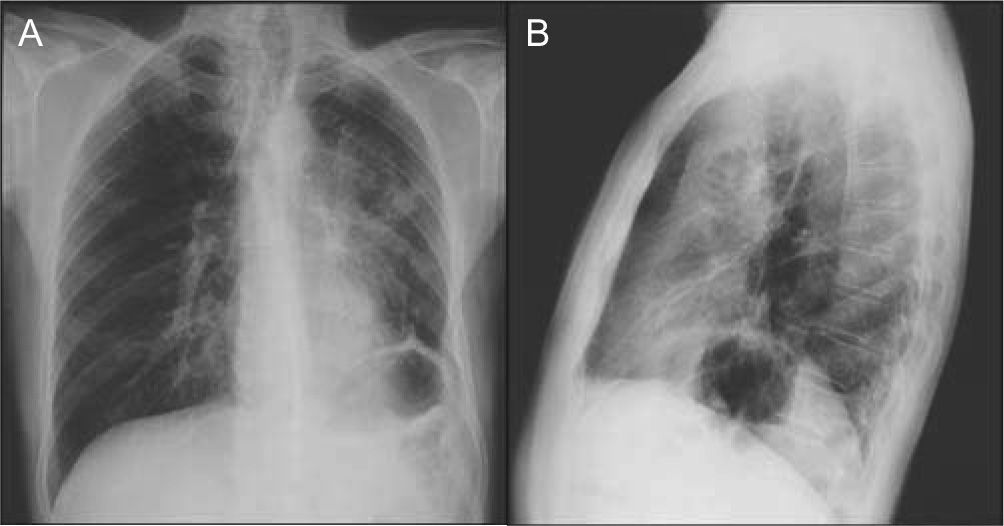
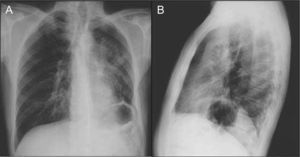
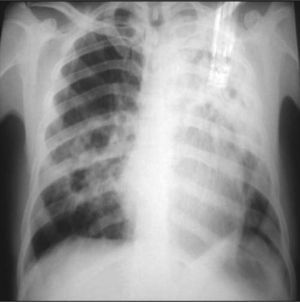
The poster anterior (PA) and left lateral chest X-ray showed heterogeneous hypotransparency with undefined contour on the left pulmonary field and elevation of the homolateral hemidiaphragm (Fig. 1).
Upon admission, the following hypotheses were considered: reactivation of pulmonary tuberculosis, community-acquired pneumonia and lung cancer. The patient began treatment with levofloxacine. However, since he remained febrile the antibiotic was changed to piperacillin/tazobactam and gentamicine.
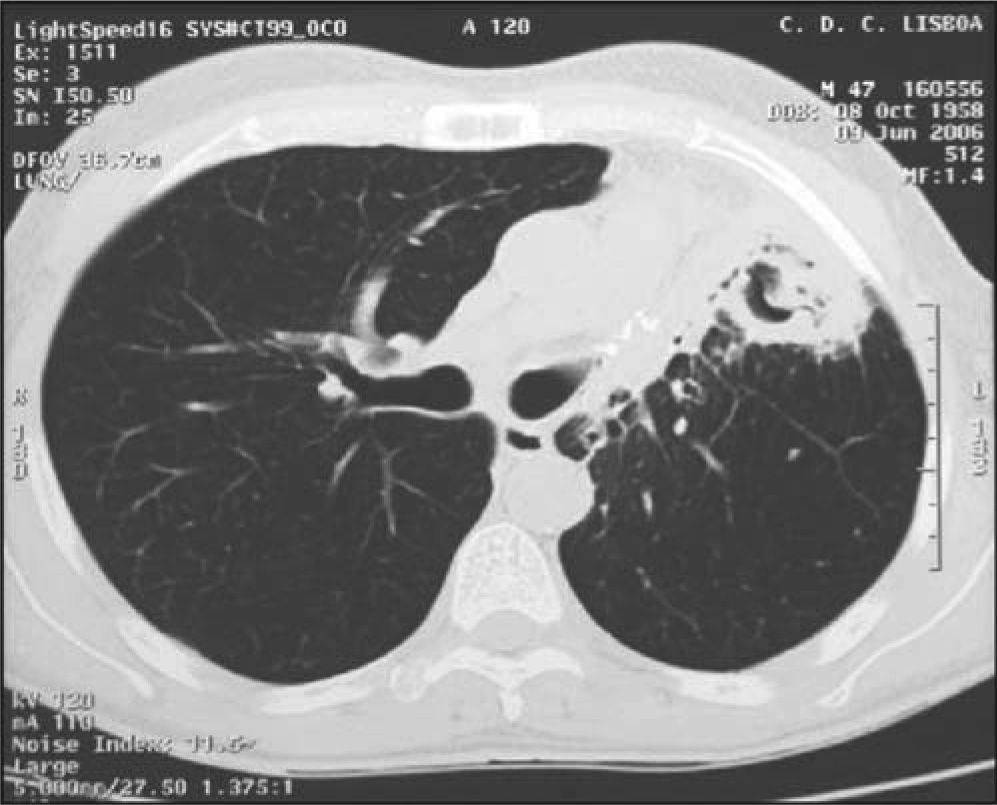
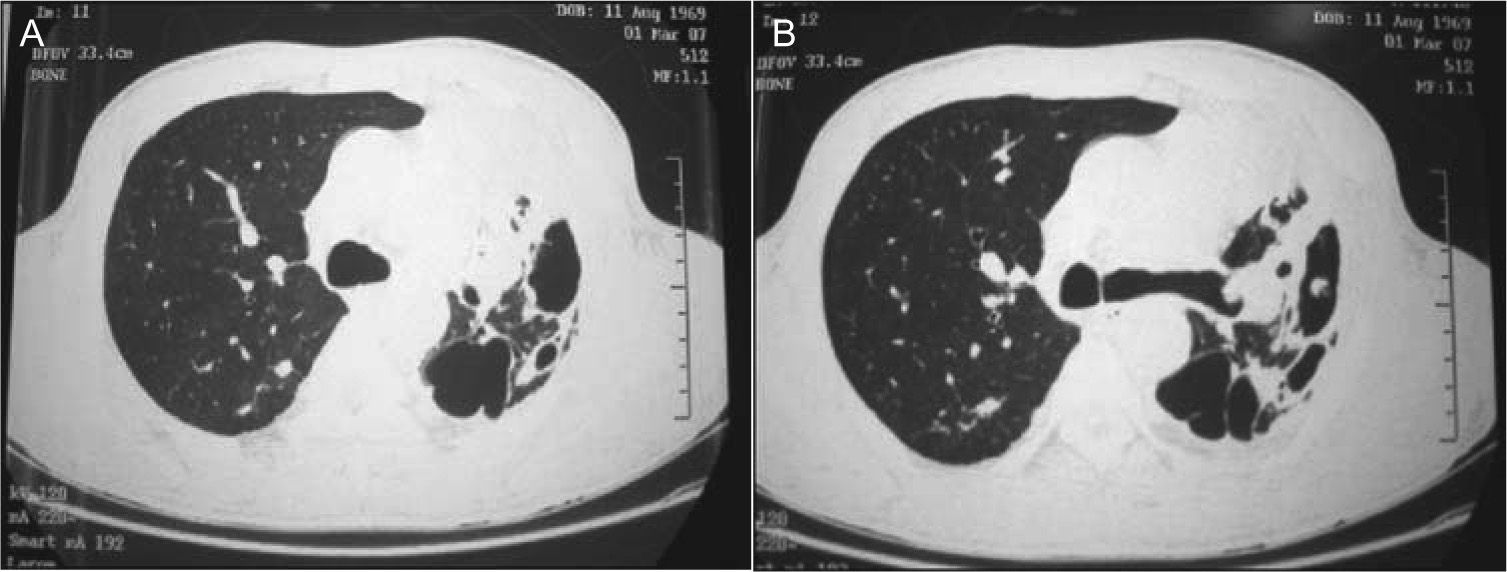
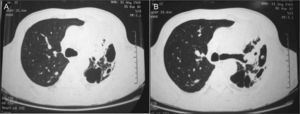
Blood cultures and sputum cultures (bacterial and mycobacterial) were all negative. Afterwards, bronchoscopy was performed, showing general inflammatory signs. Bronchoalveolar lavage (BAL) cultures (bacterial and mycobacterial) were also negative. The chest computerized tomography (CT) revealed a condensation area with a cavitation on the left pulmonary field (Fig. 2).
A significant worsening of the clinical and radiological condition was observed during hospitalization.
A new bronchoscopy was performed showing the same inflammatory signs, especially on the left lower lobe (LLL). The mycological BAL culture showed many colonies of Aspergillus fumigatus. The bronchial biopsy was compatible with a chronic inflammatory process and confirmed the presence of Aspergillus fumigatus.
The IgG for Aspergillus was 106 mg/L and the serum Aspergillus galactomannan was positive (0.51 ng/mL).
The final diagnosis was necrotizing pulmonary aspergillosis and treatment with voriconazole was started. Apyrexia was reached on the second day. The patient was discharged and prescribed with itraconazole (200 mg bid), since the oral formulation of voriconazole was unavailable at the hospital pharmacy at the time of discharge.
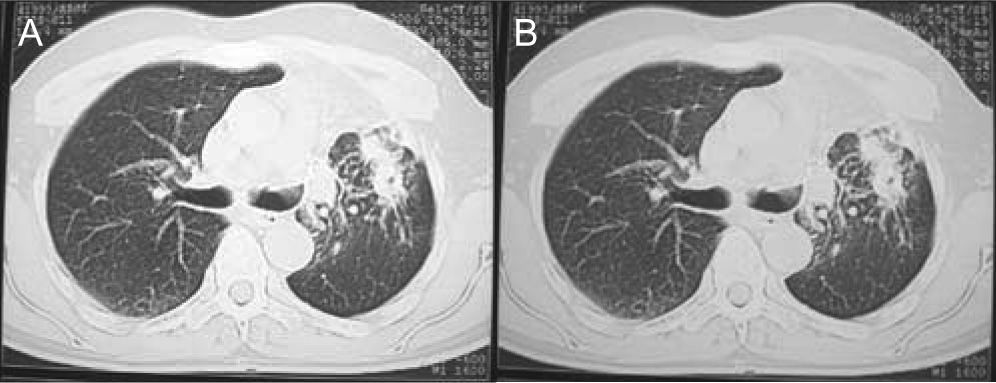
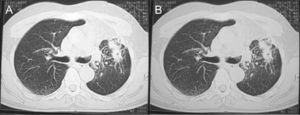
During outpatient treatment, both clinical and laboratorial improvements were registered. Residual fibrotic alterations remained in the left lung (Fig. 3). The patient followed treatment with itraconazole for 12 months.
Clinical case No. 2An afro-american 37 year-old male patient, indigent, non smoker, with significant drinking habits, was admitted with a 1-month history of cough, mucous and episodically bloody sputum, unspecific anterior chest pain, myalgias, night sweats and weakness. The patient's past medical history was as follows: pulmonary tuberculosis at 27 years of age, having abandoned treatment; atypical resection of the upper left lobe (ULL) due to a residual cavity and tuberculosis reactivation at 34 with complete treatment.
The patient had been admitted two months before due to possible acute tracheobronchitis.
Upon admission, the patient was emaciated, apyretic, hemodynamically stable, eupneic and with SpO2 of 97 % (FiO2 21 %). Pulmonary auscultation showed bilateral harsh breath sounds and increased expiration time.
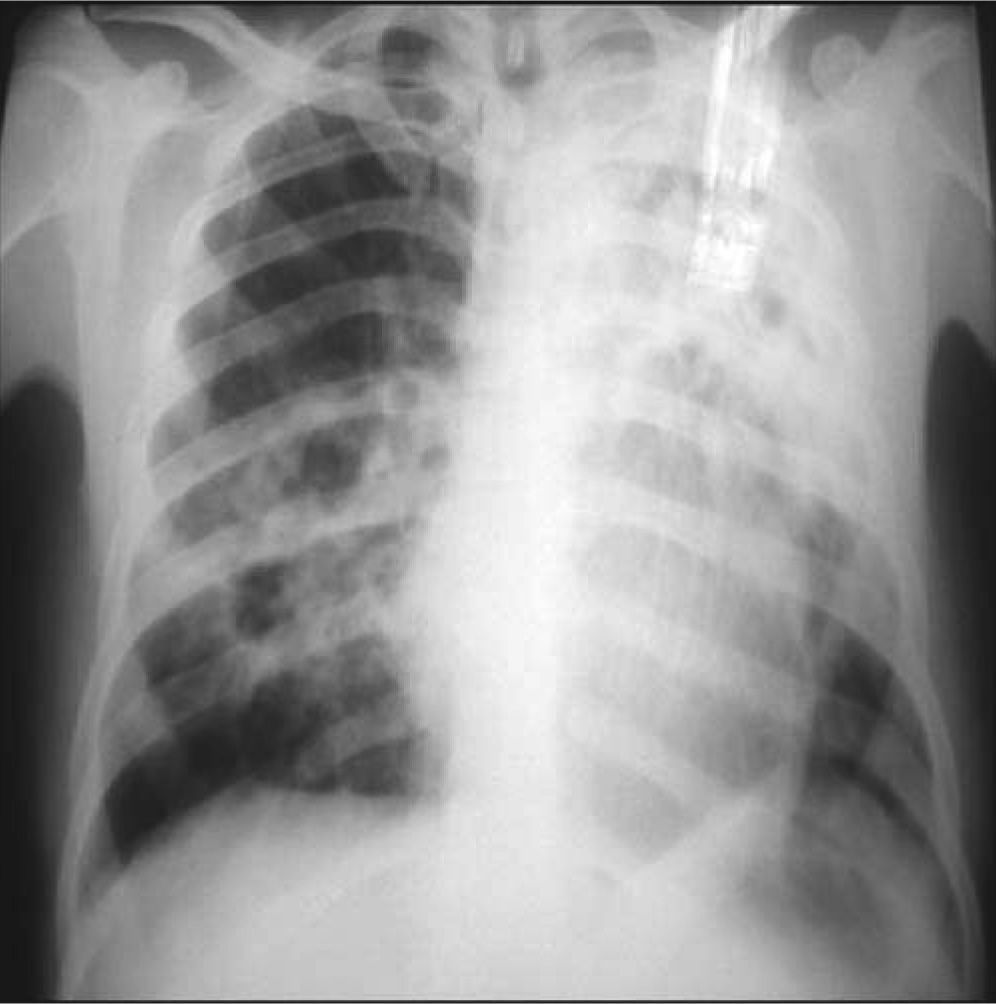
The PA chest X-ray showed diffused bilateral heterogeneous hypotransparency (Fig. 4).
Upon admission, the following hypotheses were considered: reactivation of pulmonary tuberculosis, respiratory infection in patient with sequelae of pulmonary tuberculosis and necrotizing pulmonary aspergillosis.
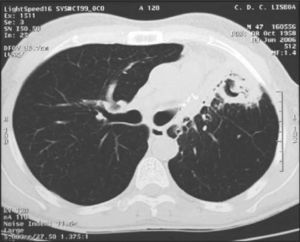
Laboratory results were: CRP, 5 mg/dL; ESR, 97 mm/first hour; HIV 1 and 2 negative. Sputum acid-fast bacilli samples were negative as well as bacterial and mycobacterial cultures. The chest computerized tomography (CT) revealed an asymmetrical thorax with decreased volume of the ULL, cavitated images and stripe hyperdensities on the LLL and the right lower lobe (RLL) and dispersed alveolar nodes (Fig. 5).
Bronchoscopy was performed, showing signs of an atypical post-resection status of the ULL and occlusion of the lingula by hard, nacarat tissue, suggesting a pathology caused by Aspergillus. The mycological BAL culture revealed some colonies of Aspergillus fumigatus. BAL Aspergillus galactomannan was positive (8.7 ng/ml). In the bronchial biopsy, the fungus was not identified. The precipitins for Aspergillus fumigatus were negative, as well as the serum galactomannan. Aspergillus fumigatus was also isolated in the sputum culture.
With a diagnosis of necrotizing pulmonary aspergillosis, the treatment with itraconazole adjusted to the patient's weight (100 mg bid) was initiated, with clinical and laboratorial improvement. The patient was discharged and referred to the outpatient department.
After sixth months of treatment, the patient was asymptomatic with complete regression of the inflammatory parametres. Chest computerized tomography (CT) revealed signs of fibrotic destruction mainly on the left lung.
By the fourteenth month of treatment, the patient remained stable, having suspended the treatment with itraconazole.
DiscussionThe necrotizing or semi-invasive pulmonary aspergillosis (NPA) is little recognized as a form of pulmonary aspergillosis, having been described for the very first time in 1981. 2,3 NPA corresponds to an indolent process of lung destruction caused by the Aspergillus fungus, generally the A. fumigatus. It is different from the invasive aspergillosis as there is no vascular invasion and dissemination to other organs.12 It mostly affects middle-aged and elder individuals. The main risk factors are: chronic obstructive pulmonary disease (COPD), sequelae of tuberculosis, pulmonary resection, radiation-induced pulmonary fibrosis, pneumoconiosis, cystic fibrosis (CF), pulmonary infarction and sarcoidosis. Other immunosuppression conditions, such as diabetes mellitus, malnutrition, alcoholism, connective diseases and prolonged corticotherapy, are also situations of increased risk.1,2,4
In general, course is insidious and the main symptoms are: cough, sputum production, chest pain, fever and weight loss.1–5 It can also be manifested through small volume or serious hemoptysis. 2,4,6
Analytically, there is an elevation of the inflammatory parameters. 1 The chest X-ray may reveal unilateral or bilateral infiltrates with or without cavitation and pleural thickness, especially in the upper lobes and in the upper segments of the lower lobes.1,2,4,5 Simultaneously, in 50 % of the cases, a conglomeration of fungal hyphae admixed with mucus and cellular debris within a preexistent pulmonary cavity, corresponding to aspergiloma.1,2 Chest computerized tomography (CT) confirms and characterizes the above described alterations.
The definite diagnosis is made through the histological demonstration of tissue invasion by the fungus and the growth of Aspergillus species in a culture.2,7 Pathologically, NPA is characterized by necrosis of lung tissue, inflammation of the cavity wall, and presence of hiphae consistent with Aspergillus species.7 Productivity of transbronchial biopsy and percutaneous aspirates is quite poor, while the highest is for biopsies by thoracoscopy or toracotomy.2,7 Due to the difficulty in confirming the diagnosis, the following diagnosis criteria were established and together are highly indicative of NPA: characteristic clinical and radiological findings, elevation of inflammatory markers (CRP, ESR) and either serological results positive for Aspergillus or the isolation of Aspergillus from respiratory samples. Active tuberculosis, non tuberculosis mycobacteriosis, cavitary histoplasmosis and coccidioidomycosis should be excluded.2,7
Galactomannan and PCR (Polymerase Chain Reaction) in bronchoalveolar lavage, as well as cutaneous sensitivity tests for Aspergillus do not have a confirmed interest in diagnosis.2,7
Once diagnosis is established, the antifungal treatment should be started immediately. 2 At present, voriconazole is the recommended drug and can be administered either orally or endovenously.8,9 The recommended dosage is 200 mg bid, reduced to half in individuals with less than 40 kg. Itraconazole can be used as an alternative in the cavitary forms. In both cases, hepatotoxicity is the main adverse reaction.8,9 The ideal treatment duration has not yet been defined and depends on the extension of the disease, the patient's response to treatment, the underlining disease and the patient's immunological condition. At times, a lifelong therapy may be required.9
Surgical treatment is reserved for young patients with localized disease or intolerance to pharmacological therapy.2
Prognosis is not well established yet. 2 In some series, survival of about 70 % was confirmed after 2 years.
Two clinical cases have been presented in which the final diagnosis was necrotizing pulmonary aspergillosis. Both patients had risk factors, namely sequelae of tuberculosis and significant drinking habits.
They displayed a clinical condition with relatively indolent progression. However, it should be pointed out that in the first case there was a progressive clinical and radiological worsening of the patient's condition during hospitalization.
As regards to diagnosis, it is important to emphasize that the patient of the first clinical case had been treated during hospitalization with piperacillin/tazobactam, which could originate false positive galactomannan results.
Due to the fact that treatment is generally quite prolonged and may present toxicity, appropriate clinical and analytical monitoring is required. Despite the fact that both patients had former drinking habits, no adverse reactions were registered, namely hepatic reactions.
Despite the fact that both patients showed a significant clinical improvement, both maintained relevant sequelae alterations.
NPA is an uncommon pathology and, frequently, difficult to diagnose. The fact that it is generally an indolent disease and that a significant percentage of patients had a prior pulmonary pathology, namely sequelae of tuberculosis or COPD, may contribute towards delayed diagnosis. This becomes even more important in the Portuguese population due to high prevalence of sequelae of tuberculosis. Therefore, it is necessary to increase the level of suspicion for this pathology, especially in patients with important sequelae alterations, and perform the recommended diagnostic tests in order to commence the correct treatment in a timely manner.