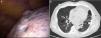
Birt–Hogg–Dubé syndrome (BHDS) or Hornstein–Knickenberg syndrome, is a rare autosomal dominant disorder, clinically characterized by skin fibrofolliculomas, pulmonary cysts, which can lead to spontaneous pneumothorax, and renal malignancy.1,2
This condition is caused by germline mutations in the tumor suppressor gene folliculin (FCLN) (OMIM#135150), located in the short arm of chromosome 17 (17p11.2).3
The clinical manifestations are widely variable which may account for BHDS being underdiagnosed.1,3
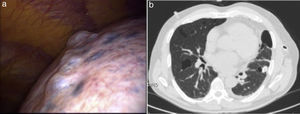
We present a case of a never-smoking, 57-year-old male, presented to the emergency department with a 3-week history of dyspnea and dry cough. His past medical history included a left spontaneous pneumothorax 20 years ago, which was conservatively managed, hypertension and chronic atrial fibrillation. On examination his chest revealed hyper-resonance and reduced breath sounds over the left lung. Chest X-ray showed a left-sided pneumothorax with complete lung collapse. Since it was his second episode, chest drainage was performed, followed by thoracoscopic pleurodesis (Fig. 1a). His chest-CT showed multiple bilateral sharply marginated pulmonary cysts (Fig. 1b). No subdiaphragmatic abnormalities were identified. On further clinical examination, our patient was noted to have pale, flat macules over his face, neck and upper trunk, for at least 20 years. There was also a family history of similar skin lesions affecting his mother. The presence of these skin lesions together with recurrent pneumothorax was suggestive of BHDS, which was further confirmed by genetic testing. This revealed a pathogenic frameshift c.573_574delinsT (p.Lys192Argfs*31) mutation in heterozygosity on exon 6 of the FCLN gene, previously described by Lencastre et al.,4 which confirms the diagnosis of BHDS.
This syndrome was first described in 1977 by Birt, Hogg and Dubé in a Canadian family in which several members had skin lesions consisting of fibrofolliculomas with trichodiscomas and acrochordons.1,2 More than 20 years later, the gene locus was mapped to chromosome 17p11.2.1–5
Nowadays, pathogenic FCLN mutations have been described in more than 200 families worldwide.1,3 FCLN is a tumor suppressor gene2 expressed in many tissues like skin, type-1 pneumocytes in the lungs and distal nephron in the kidneys.3
Cutaneous fibrofolliculoma is the most frequent clinical manifestation and consists of multiple, asymptomatic, dome-shaped pale whitish papules with 2–4mm diameters located primarily in the face, neck and upper torso.1–3 Lesions develop after 25 years in 82–92% of the affected individuals.2 The most feared complication of BHDS is renal cancer. There is a sevenfold increased risk of renal cancer compared to the general population.1,3 Lung cysts and recurrent pneumothorax can be the earliest and only manifestations of BHDS.3 Lung cysts have been described in 77–89% of BHDS affected individuals. Pulmonary function tests are usually normal despite the presence of multiple lung cysts.1,3
BHDS lung cysts are distinct from bullae and blebs. The characteristic chest CT findings are multiple lung cysts of various sizes with an irregular shape and thin wall, commonly located in the lower medial and subpleural regions of the lung.6 The pneumothorax incidence in these patients has been estimated to be 33–38%,3 that is a 50-fold increased risk compared to unaffected individuals.1–3 Recently, Johannesma et al. stated that probably 5–10% of (apparentely) primary spontaneous pneumothorax are caused by BHDS.7
We established our patient's diagnosis according to the criteria proposed by Menko et al. which include BHDS clinical manifestations and positive DNA testing result.1
If genetic testing is positive, family members should be referred for genetic counseling and FCLN sequencing.1 Whenever BHDS is suspected, chest CT is recommended to identify lung cysts or the presence of pneumothorax.2 As pneumothorax recurrence rate is high, treatment differs from the one recommended for primary spontaneous pneumothorax, and according to some authors pleurodesis should be considered as the first line treatment option.6
Pneumococcal and annual influenza vaccination are recommended, as well as periodic pulmonary function testing if abnormal lung function is noticed.6
Family members at risk of BHDS should start renal tumor screening with MRI when reaching the age of 21 years, and preferably every 3 years thereafter. Once a renal mass is detected, screening should become annual until the mass has 3cm in diameter, surgery being recommended afterwards.2
Cutaneous lesions in BHDS are asymptomatic and hence treatment is offered for psychological and cosmetic reasons only.1,2
This is to our knowledge the second reported case of BHDS in Portugal. Our aim is to alert to its existence in order to allow for early detection and prevention of the more serious complications, such as renal cancer, both in presenting patients and in relatives at-risk.
Conflicts of interestThe authors have no conflicts of interest to declare.