Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrosing interstitial pneumonia of unknown cause that primarily occurs in older adults.1,2 It is characterised by the histopathologic and/or imaging pattern of usual interstitial pneumonia (UIP), typically with peripheric involvement and a cranio-caudal gradient.1,2
The reason for the peripherical predominance, which generally starts at the posterior bases of the lower lobes and then progressively extends in a caudal-cranial mode, remains unknown.1-3 One hypothesis suggests that the concurrent action of cell senescence, genetic predisposition, exposure to cigarette smoke and mechanical stress caused by respiratory lung movements leads to the localised exhaustion of the tissue renewal capacity and, eventually, alveolar loss and abnormal lung remodelling.3-5
We present the case of a patient who developed a probable UIP pattern after undergoing a left lower lobe (LLL) lobectomy due to a tumour. Interestingly, the patient's remaining left upper lobe (LUL) exhibited reticulation and traction bronchiectasis that resembled the features usually observed in the lower lobes, which indicated the possible relevance of mechanical tension theory. The patient consented to the publication of this clinical case.
A 70-year-old male former smoker underwent a computed tomography (CT) scan that revealed a 13-mm LLL cavitated nodule (Fig. 1A), which the CT transthoracic biopsy indicated to have features of squamous cell lung carcinoma. After staging, the patient underwent an LLL lobectomy with lymph node removal. The pathologic assessment revealed a stage I tumour (pT1b N0 R0) according to the American Joint Committee on Cancer criteria (7th edition). No other histologic features related to interstitial lung disease were identified. The patient was admitted for follow-up and no adjuvant therapy was administered.
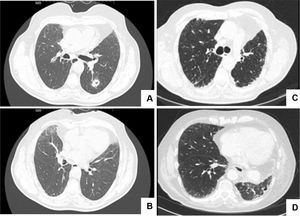
CT scans before and after left lower lobe lobectomy (LLL).Fig. 1A shows a 13 mm cavitated nodule in the LLL, posteriorly confirmed to correspond to a squamous cell lung carcinoma; no signs of interstitial lung disease were present in the lower lobes (Fig. 1B). Fig. 1C and D shows bilateral peripheric and basal reticulation and traction bronchiectasis in the inferior region, more evident in the left upper lobe, 21 months after LLL lobectomy.
During follow-up, the patient remained stable, with his only respiratory symptom being exertional dyspnoea (Modified Medical Research Council Dyspnoea Scale score = 1). However, the protocolled 18-month CT scan revealed a peripheral and basal reticulation with traction bronchiectasis, which persisted at the 21-month assessment and exhibited slight progression at the 24-month evaluation. Although bilateral, these imaging features were more evident in the inferior portion of the LUL (Fig. 1B).
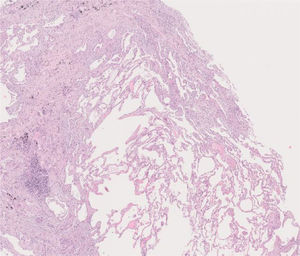
After a multidisciplinary team (MDT) discussion, a cryobiopsy of the patient's LUL was performed, which revealed the replacement of portions of alveoli by irregular fibrous scars in a patchwork pattern and fibroblast foci. The scars were composed of collagen with scant chronic inflammation. Moreover, their distribution was predominantly paraseptal. The adjacent pulmonary parenchyma presented minimal interstitial inflammation. These aspects were compatible with a UIP pattern (Fig. 2).
Histology of the cryobiopsy performed in the left upper lobe. At this magnification, normal alveolar tissue is surrounded by portions of alveoli replaced by irregular fibrous collagenous scars, with a predominantly paraseptal distribution. At higher magnifications, fibroblast foci were seen. The adjacent parenchyma has minimal interstitial inflammation. The aspects were compatible with usual interstitial pneumonia.
Apart from being a former smoker, the patient did not report any relevant exposures and his autoimmune panel was within the normal range. The final diagnosis of IPF was established during an MDT meeting and the patient was prescribed 801 mg of pirfenidone three times a day.
Following an LLL lobectomy, the development of an UIP pattern mainly affecting the LUL strengthens the potential relevance of mechanical tension in relation to the manifestation of fibrosis. Pre-existing factors such as ageing, exposure to environmental pollutants (e.g. smoking habits) and unknown genetic abnormalities may promote the occurrence of IPF after an insult that increases the magnitude of the respiratory mechanical stress.3,4 Mechanical forces can be particularly concentrated in the peripheral and basal anatomical parts of the lung, thereby triggering the formation of microscopic damage to the alveolar structure, which may cause repetitive small scarring events (fibroblast foci) and, eventually, honeycomb changes.4,5 Additionally, a subsequent increase in the extracellular matrix stiffness and the progressive scarring of the lung tissue significantly change the respiratory mechanics, rendering the lung more fragile and more exposed to non-physiological stress during both spontaneous breathing and mechanical ventilation, which can promote progressive lung damage and the dysregulation of mechano-transduction and tissue repair.4,5
Carloni et al. demonstrated that mechanical stress during lung inflation is heterogeneously distributed in different anatomical parts of the lung parenchyma and, further, that these overloaded regions correspond to those involved in early IPF lesions.5 More recently, Wu et al. showed that the loss of Cdc42 function in alveolar stem 2 cells (AT2) causes periphery-to-centre progressive fibrosis.6 It has been established that the application of mechanical stretch to AT2 cells activates the transforming growth factor beta (TGF-β) pathway, which induces a disturbance in the homeostatic microenvironment, leading to aberrant wound healing and promoting the fibrotic process.6,7 The authors also showed that Cdc42-null AT2 cells in post-pneumonectomy and untreated aged mice could not regenerate new alveoli, resulting in sustained exposure to elevated mechanical tension, which activated TGF-β signalling.6 This study provides new insights that emphasise the association between mechanical tension and progressive lung fibrosis.6
The precise recognition of this physiologic process is vital, as it can inform the identification of preventive measures to avoid fibrosis and suggest new therapeutic paths.