Nitric oxide is a gas produced in the airways of asthmatic subjects and related to T2 inflammation. It can be measured as fractional nitric oxide (FeNO) in the exhaled air and used as a non-invasive, easy to evaluate, rapid marker. It is now widely used in many settings to determine airway inflammation. The aim of this narrative review is to report relationship between FeNO and the physiopathologic characteristics of asthmatic patients. Factors affecting FeNO levels have also been analysed as well as the impact of corticosteroid, target therapies and rehabilitation programs. Considering the availability of the test, spreading this methodology to low income countries has also been considered as a possibility for evaluating airway inflammation and monitoring adherence to inhaled corticosteroid therapy. PubMed data search has been performed restricted to English language papers. Research was limited to studies in adults unless studies in children were the only ones reported for a particular issue.
This revision could be useful to summarize the role of FeNO in relation to asthma characteristics and help in the use of FeNO in different clinical settings particularly in low income countries.
Asthma is a heterogeneous disease usually characterized by airway inflammation.1 Inflammation characterizes most of the steps of the disease, and is strictly associated with airway remodelling.2 Skewing towards type 2 inflammation is usually a characteristic of asthma with eosinophils represented as the most common cells recruited in the airways.3 This type of inflammation is treatable with inhaled corticosteroids (ICS), associated with long acting beta 2 agonist (LABA).4 However, severe asthmatic patients at the top of ICS + LABA prescriptions experience frequent exacerbations, which need oral corticosteroids.1 Introduction of monoclonal antibodies targeting IgE, IL-5, IL-5R and IL-4Rα successfully reduce T2 inflammation in these patients, avoiding in most of the cases, oral corticosteroid use.5
The evaluation of inflammation in asthmatic patients may be useful at time of diagnosis, in order to define the inflammatory pattern of the patient, and even more useful during the follow-up to assess patient’s adhesion to therapy, response to treatment and necessity of step-up or down of therapy. Furthermore, in patients with severe asthma not controlled by high dose ICS, the evaluation of the type of airway inflammation is useful to select candidates for monoclonal therapy.1
Many efforts have been focused in recent years on trying to detect T2 inflammation with distinct biomarkers in different specimens. Biopsies and bronchoalveolar lavage were the first used to assess airway inflammation but, due to the invasiveness of the procedures, their use is limited to research or to special cases. Induced sputum and fractional exhaled nitric oxide (FeNO) measurements are the most reliable non-invasive procedures to assess T2 airway inflammation.6,7 Induced sputum allows us to monitor airway inflammation in asthmatic patients and to reduce exacerbations when used to modulate ICS therapy, evidence A.1 Although reproducible and standardized by International guidelines,8,9 induced sputum diffusion is relatively time consuming and limited to specialized centres. Efforts have been made to simplify this technique and spread it in less developed countries.10 Nitric oxide (NO) is a gas produced in the airways and detectable as exhaled NO. Its production increases in the airways when T2 inflammation is present; therefore, measurement of FeNO is a non-invasive technique to highlight T2 airway inflammation. FeNO evaluation is certainly more widespread in different clinical settings than induced sputum because it does not require particular equipment, apart from the specific analyser. Furthermore, induced sputum technique is time-consuming, needs staff training not only for the collection but also for the processing of samples.
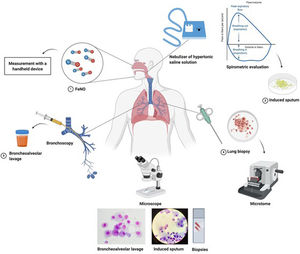
In recent decades, different studies evaluated the correlation between FeNO and sputum eosinophils in order to use this biomarker as a surrogate for sputum eosinophils even in low income countries. The correlation between these two biomarkers is significant but weak since sputum eosinophils and FeNO only partially share T2 mechanisms of inflammation.11 Blood eosinophils have also been used in recent years as surrogate marker of sputum eosinophils12 with different accuracy with respect to FeNO when sputum eosinophils were used as “gold standard” for eosinophilic airway inflammation. Fig. 1 summarizes the most consolidated and widespread methodologies to assess airway inflammation. Exhaled breath condensate has been widely used for research in airway inflammation with the evaluation of different mediators such as H2O2, 8-isoprostane, adenosine, pH and leukotrienes, but as reported by the European Respiratory Society (ERS) task force for exhaled biomarker in lung diseases, standardization of collection, storage and evaluation procedure are needed for this methodology.13
Most used methodologies to assess airway inflammation, from the least to the most invasive ones. 1. FeNO can be measured with a handled device, 2. Induced sputum needs an ultrasonic nebulizer, serial spirometric evaluation during the inhalation period to avoid excessive bronchoconstriction and sample processing in the laboratory, 3. Bronchoalveolar lavage is performed during a broncoscopy procedure and needs a processing of the sample in the laboratory, 4. Biopsy, with close or open methods, is the most invasive procedure used only in selected cases, less frequently to evaluate airway inflammation. Figure was created with Biorender.com.
FeNO has recently been the topic of review papers, but to the best of our knowledge, this is the first review trying to summarize the relationship between FeNO, asthma characteristics, interfering factors and therapeutic approaches, rehabilitation programs included. It also considers the scaling up of this methodology to low income countries.
MethodsWe conducted a literature search in PubMed to find studies focused on asthma characteristics and FeNO in order to evaluate which the relationships reported between this biomarker and characteristics of the disease. The search terms were: FeNO (or fractional exhaled nitric oxide) AND asthma in all fields of search AND hyperreactivity OR hyperresponsivness, OR airway remodelling, OR infection, OR air pollution, OR smoking, OR exercise, OR comorbidities (atopic dermatitis, nasal polyposis, etc.), OR occupational asthma, OR developing countries, OR rehabilitation programs. We considered English papers from 1994 to February 2020 and studies in adult subjects, unless those in children were the only reported on a particular issue.
ResultsFeNO definitionNitric oxide is a gas present in the airways and detectable as fractional nitric oxide (FeNO) in the exhaled air. High FeNO levels are found in a sub-group of asthmatic patients with T2 inflammation. Inducible nitric oxide synthases (iNOS) are the enzymes responsible for the increase of NO in the airways due to inflammation. Analysis of bronchial epithelial different gene expression in asthmatic patients revealed different clusters of subjects with high or low FeNO levels suggesting different molecular pathways.14 Measurement of FeNO at different flows allows us to evaluate the amount of this gas produced by central and peripheral airways and to obtain selective information regarding inflammation at different sites of the airways. Differences between electrochemical and chemiluminescence based analysers in FeNO levels have recently been reported,13 proposing an equation to convert one level to another.15
FeNO use in asthmaIncreased FeNO levels in asthmatic patients were reported many years ago.16 American Thoracic Society (ATS) /ERS guidelines summarized data on FeNO measurement showing that FeNO levels >50 ppb are representative for T2 inflammation in adults, while FeNO<25 ppb for lack or suppression of it, intermediate levels should be cautiously evaluated considering possible factors lowering (smoking habit) or increasing (atopy) FeNO levels.17 The positive predictive value (PPV) for FeNO>25 ppb to predict sputum eosinophils >3% is 45% while for FeNO > 50 ppb PPV increases to 77%. Negative predictive value of FeNO is probably more useful (88% and 83% respectively),18 particularly considering that application of these cut-offs in real life run into many subjects with intermediate levels (25–50 ppb).18 FeNO levels relate more closely to the risk of disease exacerbations than to disease severity. The ability of FeNO to predict airway inflammation varies as opposed to airway calibre, therefore, FEV1 reduction with involvement of peripheral airways can be associated with low FeNO levels even if airway eosinophilic inflammation is high,19 on the contrary, bronchial obstruction limited to central airways can be associated with increased FeNO levels. FeNO levels may identify non-smoker patients with not well-controlled asthma while sensitivity of FeNO seems lower than evaluation through ACT and ACQ.20
In mild allergic asthma FeNO levels decrease after a specific inhalation challenge together with bronchoconstriction, they return to normal levels after 8 h and again increase 24 h after the challenge.21
FeNO levels correlate with asthma exacerbations in severe asthmatic patients independently of blood eosinophils and serum periostin levels.22,23 Since cough is a frequent asthma symptom, which can be associated with high T2 inflammation, cough variant asthma can be suspected when cough and high FeNO levels are present.24
Atopy is associated with increased FeNO in children while in adult subjects FeNO levels increase when allergic rhinitis is present.25 Age and height are also positively correlated with FeNO levels.26 FeNO levels are associated with a rapid lung decline in patients with difficult to treat asthma.27
FeNO and characteristics of asthmaBronchial hyperresponsivnessBronchial hyperresponsivness (BHR) is a characteristic of asthmatic patients not always associated with T2 inflammation.28 Increased FeNO levels, independently of increased blood eosinophils, correlate with BHR in young asthmatic patients and the simultaneous increase of FeNO and blood eosinophils increases the risk of BHR.29 Very high FeNO levels (> 100 ppb) are strong predictors of bronchial hyperreactivity in Asian patients with suspected asthma.30 Furthermore, FeNO has been proposed as predictive marker of bronchial hyperreactivity to both mannitol and bradykinin in asthmatic patients.31,32
Bronchial reversibilityDifferent stimuli, both specific and non-specific can trigger bronchoconstriction in asthmatic patients.1 FeNO levels might be lower than real when bronchoconstriction is present.33 As recommended by ATS/ERS guidelines, FeNO should be measured before spirometric manoeuvres.17 Albuterol inhalation in steroid-naïve patients, determined increased FeNO levels.34 In a cohort of non-smoking asthmatics, baseline FeNO levels correlated with bronchial reversibility,35 and with change in FEV1 occurred after a reversibility test in patients treated with ICS/LABA.36 In subjects with asthma symptoms but with absence of reversibility, FeNO >32 ppb can predict positivity to methacholine test with high specificity (85 %) and lower sensitivity (47%).37
Airway remodellingRemodelling is a complex and multifactorial event characterized by increased thickness of the reticular epithelial membrane, hypertrophy of airway smooth muscle cells, hyperplasia of goblet cells and angiogenesis processes.38 Since remodelling is often present together with inflammation, it is rather difficult to evaluate the contribution of NO directly to the remodelling process. High FeNO levels could be related to increased bronchial wall NO concentration due to airway inflammation or altered bronchial diffusivity of NO due to remodelling.39
Many years ago, Mahut et al. found in children with refractory asthma that alveolar nitric oxide correlated with TGF-β, a well-known pro-fibrotic cytokine, reticular membrane thickness, tissue inhibitor metalloproteinase (TIMP)/metalloproteinase 9 in BAL and with MEF 25-75.40 Structural changes in the airways of asthmatic patients seem to be correlated with FeNO levels. Alveolar NO represents the production from the seventeenth to the twenty-third generation of bronchi, while central airway NO is produced from the first to the sixteenth generation.41 In adult asthmatic patients FeNO levels correlate with wall thickening in the central area, suggesting a role particularly with the remodelling of this tract.41 Therefore, differentiation between NO derived from central or peripheral airways could be useful when airway remodelling is studied. Treatment with corticosteroids after exacerbations slightly decreased FeNO levels without affecting bronchial wall thickening in moderate/severe persistent asthmatics,42 confirming an active role of ICS on the amount of NO produced by bronchial walls and associated to inflammation.
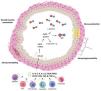
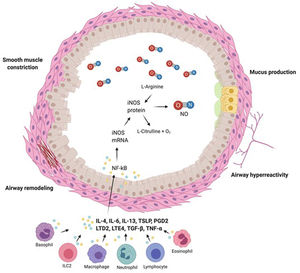
Characteristics of airways in asthmatic patients and FeNO levels are summarized in Fig. 2
Airways of asthmatic patients are characterized by reversible bronchoconstriction, bronchial hyperresponsiveness and airway remodelling. Nitric oxide (NO) present in the airways is mainly produced by epithelial cells as a consequence of the activation by cytokines of the transcription factor NK-kB which stimulates production of inducible nitric oxide syntetase (iNOS), the enzyme responsible for NO production. Figure was created with Biorender.com.
Asthmatic patients often have one or more comorbidities and FeNO levels could change depending on how comorbidities affect airway inflammation (Table 1). The prevalence of chronic rhinosinusitis with nasal polyposis is high in severe asthmatic patients (40.6%).43 FeNO is a good predictor of nasal polyposis in severe asthmatic patients even when blood eosinophils are normal or low.44
Relationship between FeNO levels and comorbidities in asthmatic patients.
| Comorbidity | FeNO | Patients | Advantage of FeNO evaluation | Ref. # |
|---|---|---|---|---|
| Chronic rhinosinusitis with nasal polyposis | Levels increased in asthmatic patients with chronic rhinosinusitis with nasal polyposis | Severe asthmatics (n = 695); nasal polyposis (n = 282), 2.5% smokers; ICS and OCS treated | To select patients with nasal polyposis even when blood eosinophils are low | 43 |
| Levels associated with nasal polyposis | Severe asthmatics (n = 93); nasal polyposis (n = 28); 12% smokers; all ICS treated, 30% OCS treated | 44 | ||
| Obesity | Levels not affected by increased BMI | Severe asthmatics (n = 286); obese (n = 96); smoking history not reported; most of the patients in corticosteroid treatment | Help in distinguishing when obesity is a comorbidity of asthma or the cause of lung function impairment | 48 |
| High FeNO is associated with more obstructive changes in obese patients | Asthmatics (n = 472); obese (n = 248); current smokers (n = 56); ICS treated (n = 82) | 49 | ||
| Gastroesophageal reflux disease (GERD) | Low FeNO associated with GERD symptoms | Asthmatics (n = 248), smoking history not reported, treated with ICS (n = 246); proton pump inhibitory therapy (n = 61). | Presence of high FeNO in GERD patients suggests eosinophilic inflammation due to other causes | 50 |
| Obstructive Sleep Apnoea Syndrome (OSAS) | Levels not affected by OSAS | ICS treated asthmatics (n = 60), Smoking history not reported | Presence of high FeNO in OSAS patients suggests eosinophilic inflammation due to other causes | 51 |
| Allergic rhinitis | FeNO increases with active allergic rhinitis, nasal steroid treatment decreases FeNO levels | Asthmatics (n = 520); with allergic rhinitis (n = 348), No current smokers, 397 never smokers. Nasal mometasone furoate treated (n = 40) | In asthmatics with allergic rhinitis high FeNO alerts to uncontrolled nasal symptoms | 52 |
| Bronchiectasis | Low FeNO was associated with presence of bronchiectasis | Uncontrolled moderate to severe asthmatics (n = 398); with bronchiectasis (n = 113), non smokers, ICS treated | Higher FeNO levels (> 20.5 ppb) suggest lower probability of bronchiectasis | 55 |
ICS = inhaled corticosteroid therapy, OCS = oral corticosteroid therapy.
Obesity is a comorbidity with impact on lung function, particularly in asthmatic patients and any effort towards weight loss could improve disease severity and quality of life.45 Adipokines produced by adipose tissue determine a low grade of inflammation, which also affects the lungs.46 This type of inflammation, mainly characterized by increased IL-6 and IL-8 levels, has a relationship with lung functions in obese asthmatic patients.47 In obese asthmatic patients, FeNO evaluation does not seem to be affected by obesity48 and can be useful in defining the type of inflammation and the cause of respiratory symptoms. High FeNO levels were found in obese asthmatics with an eosinophilic inflammation, which can be the trigger of bronchial symptoms while low FeNO levels are the result of restrictive rather than obstructive functional changes mainly caused by obesity.49
Gastroesophageal reflux disease (GERD) is a common comorbidity of asthma, mildly associated with neutrophilic airway inflammation without impact on FeNO levels50 in subjects treated with controller medication for asthma. A subgroup of asthmatic patients is complicated by obstructive sleep apnoea that did not seem to impact on FeNO levels.51 Patients with allergic rhinitis showed evidence of peripheral airway inflammation with increased FeNO levels,52 impairing the clinical interpretation of FeNO levels.53 Asthmatic patients with active allergic rhinitis had increased FeNO levels, which decreased together with Asthma Control Test (ACQ) when patients were treated with nasal corticosteroids.54 Low FeNO levels in uncontrolled moderate/severe asthmatic patients can predict the presence of bronchiectasis.55
Factors interfering with FeNO levelsSmokingAcute and chronic smoking reduces FeNO levels26,56 suggesting stopping smoking at least one hour before FeNO evaluation.17 A recent systematic review on FeNO in smoking asthmatic subjects concluded that FeNO levels are decreased in smoking compared to non-smoking subjects but still higher than in smoking controls; however, due to the uncertainty of the published results, caution is needed to interpret FeNO levels in smoking asthmatics.57
Viral or bacterial infectionsControversial findings have been published regarding FeNO levels in asthmatics during or after viral/bacterial infections. Infections are frequently the trigger of acute asthma exacerbations; FeNO levels do not seem to distinguish viral from non-viral asthma exacerbation.58 Malka J et al., found that asthmatic children with exacerbations had lower FeNO levels when PCR for rhinovirus was positive,59 while asthmatic children with cold like symptoms and negative viral nucleic acid/rhinovirus copies had increased FeNO suggesting airway T2 inflammation due to other causes such as low compliance, high sensitizer exposure, etc.60 Asthmatic subjects, both young adults and adolescent, without infection symptoms but positive for human rhinovirus do not have increased FeNO and blood eosinophils compared to negative asthmatic subjects.61
Iikura et al. reported that adult asthmatic patients with viral infections had lower FeNO levels during asthma exacerbation than non-infected subjects,62 while patients with bacterial infections, mainly due to S. pneumonia and H. influenzae, had comparable levels of FeNO to non-infected patients both during exacerbation and in stable conditions.62,63
Exposure to air pollutionMany studies underlined the association between air pollution and airway inflammation. The relationship between air pollution exposure and FeNO has been mainly studied in children, with a correlation found between ultrafine particle concentrations (<0.1 μm) and FeNO levels in atopic subjects.64 Unselected children exposed to industrial pollution presented increased odds ratio of having higher FeNO (>30 ppb).65 Exposure to nitrogen dioxide, as indicator of air pollution, has been associated with FeNO levels in children living in Australian cities, suggesting a possible detrimental inflammatory effect of this compound in subjects with genetic and epigenetic susceptibility of iNOS.66 Acute exposure to traffic air pollution did not affect FeNO production in adult subjects with mild asthma.67 FeNO levels were associated with concentrations of PM 2.5 in unselected older women, some of them with chronic inflammatory airway conditions and exposed to air pollution.68
Indoor pollution can also cause/affect airway inflammation. The presence of dampness, moulds, particularly Aspergillus versicolor DNA, in schools was associated with high FeNO in students.69 Recently a correlation between FeNO levels and indoor mycobiome was found in severe asthmatic patients.70
Exposure to low doses of Dermatophagoides pteronissinus, was associated with increased FeNO in atopic patients with mild asthma without worsening of symptoms.71
Occupational exposureWork exposure to high or low molecular weight agents can induce, in predisposed subjects, occupational asthma.72 Moreover, work substances even if not directly responsible for asthma can determine increase in airway inflammation and work exacerbation of pre-existing asthma. FeNO is considered a non-invasive methodology to assess airway inflammation in the diagnostic work-up of occupational asthma.73 Its use in this context can be useful when combined with specific inhalation challenge, when evaluated at and away from work. Among apprentices, FeNO levels are associated with sensitization and its increase after work exposure related to the incidence of bronchial hyperreactivity.74–76
Physical exercisePhysical exercise can affect symptoms and airflow limitation in asthmatic patients. FeNO levels decreased after acute exercise of moderate intensity particularly at low temperatures in asthmatic or allergic patients.77 FeNO does not predict a positive exercise test in children with respiratory symptoms during physical activity.78 Exercise, such as aerobic training, in patients with moderate or severe persistent asthma can reduce FeNO and sputum eosinophils in patients with worse airway inflammation.79 As to the cumulative effect of swimming activity, Škrgat et al. showed that FeNO and blood eosinophils did not differ between intensive training and stopping period in non-asthmatic adult competitive swimmers.80 The decrease of FeNO, as acute effect, reported hours after the swimming activity has been hypothesized to be caused by either a direct neurogenic response or an inhibitory effect on iNOS.81
FeNO versus other biomarkers of T2 inflammationFeNO levels slightly correlated with airway eosinophils evaluated through induced sputum,82 bronchoalveolar lavage83 while contrasting results were found with biopsies.32–84
Most of FeNO in the airways is produced by iNOS stimulated by IL-4/IL-13 signalling during inflammatory processes. T2 inflammation in asthma is characterized by an increase of different cytokines reflecting the activation of specific immunologic pathways; FeNO levels seem to correlate better with IL-4 and IL-13 while eosinophils with IL-5. IL-13 is one of the main drivers of iNOS activation and consequent FeNO production.85 In the meantime, IL-13 activates eosinophils and favours eosinophil extravasation through the up-regulation of adhesion molecules in the endothelium. Furthermore, IL-13 increases in the airways the production of chemokines able to bind the CCR3 receptor and chemotactic for eosinophils.86
FeNO and blood eosinophils can be used independently to predict airway inflammation; they did not correlate each other in uncontrolled asthma87 and mildly correlated in non-smoker asthmatics.88 A weak correlation between blood eosinophils and FeNO levels was found in young asthmatic patients, and subjects with simultaneous increase of FeNO and blood eosinophils had higher bronchial hyperreactivity and low asthma control.29 However, discrepancies between FeNO levels and blood eosinophils have been reported in subgroups of asthmatic patients.89 In non-smokers with mild-moderate persistent asthma, FeNO had high sensitivity in predicting eosinophilic asthma compared to blood eosinophils and serum eosinophil cationic protein (ECP).90 Subjects with high FeNO and low blood eosinophils had higher numbers of sensitization to inhaled allergens than subjects with low FeNO.91 FeNO as single measurement, failed to predict persistent blood eosinophilia in patients with new-onset asthma compared to single blood eosinophil evaluation.92 Blood eosinophils have higher sensitivity for sputum eosinophilic inflammation than FeNO and ECP in asthmatic patients treated with inhaled corticosteroids.93 Considering recent findings on different mechanisms driving eosinophilic inflammation and FeNO, it is not surprising that blood eosinophils prove more sensitive as surrogate marker of sputum eosinophils than FeNO in moderate-severe asthmatic patients.85
FeNO and total serum IgE are elevated in asthmatic patients, particularly in the atopic ones and they weakly correlate.94 FeNO was reported superior to total IgE and equal to blood eosinophils in predicting sputum eosinophilia in different subset of patients with adult onset asthma, independently to smoking history, atopy or disease severity.95
Periostin, an extracellular matrix protein, reflects activation of T2 mechanisms.96 In a study of asthmatic patients treated with the anti IL-13 lebrikizumab, subjects with high serum periostin showed improvement in lung functions compared to patients with low levels97 but these results were not confirmed in subsequent studies.98 FeNO and periostin can be simultaneously increased in asthmatic patients and in this case, they can identify severe T2/eosinophilic airway inflammation.99 When FeNO was used together with periostin and peripheral blood eosinophils to ameliorate the sensitivity of biomarkers, the combination failed to increase the predictive value for asthma exacerbations.22
In a recent analysis from the U-BIOPRED study group, serum periostin failed to predict a T2 response of airway epithelial cells compared to FeNO, blood and sputum eosinophils in asthmatic patients.11 During acute severe exacerbations, T2 biomarkers peak but mechanisms, which induce their increase are differently sensitive to steroid therapy. Semprini R. et al., demonstrated that in asthmatic patients evaluated after treatment for severe exacerbations, FeNO decreased 2 weeks, serum periostin 1 week and blood eosinophils 1 day after oral steroid intake.100 In symptomatic patients despite maximal ICS dose, serum periostin was the best predictor of airway eosinophilia compared to FeNO and blood eosinophils.101 In smokers with less controlled asthma, blood eosinophil evaluation is the most accurate in predicting airway eosinophilic inflammation compared to FeNO, IgE, serum periostin and IL-13.102
A recent position paper of the European Academy of Allergy and Clinical Immunology evaluating the clinically applicable biomarkers for asthma, recognized FeNO as the best point-of care biomarker to identify T2 endotype in asthma.103Table 2 summarizes the relationship between FeNO and other biomarkers of T2 inflammation when used to predict high airway eosinophils.
Accuracy of different T2 biomarkers in reflecting sputum eosinophila considered as sputum eosinophils higher than 3.0%, 2.5% or 2.0%.
| Ref.# | Airway eosinophilic inflammation (sputum eos) | Asthmatic Patients | Smokers | ICS | FeNO | Blood eosinophils | Serum periostin | Serum Total IgE | Serum ECP |
|---|---|---|---|---|---|---|---|---|---|
| 87 | 2.5% | Adult uncontrolled (n = 75) | yes | yes | 0.71(67.3−37.9) | 0.73 (61.5−78.3) | ND | ND | ND |
| AUC (sens %-spec %) | AUC (sens %-spec %) | ||||||||
| 90 | 3.0 % | Adult mild or moderate (n = 124) | no | no | 0.85 (0.74−0.97) | 0.92 (0.84−0.99) | ND | ND | 0.75 (0.87−1.0) |
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |||||||
| no | yes | 0.73 (0.55−0.91) | 0.70 (0.47−0.93) | ND | ND | 0.51 (0.27−0.76) | |||
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |||||||
| 93 | 3.0% | Adult moderate, severe External validation (n = 110) Replication cohort (n = 37) | no | yes | 0.78 (0.66−0.89) | 0.89 (0.81−0.96) | 0.55 (0.43−0.67) | ND | ND |
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |||||||
| Non-significant | |||||||||
| 2.0% | 0.79 (NR) | 0.88 (NR) | NR | ND | ND | ||||
| AUC (95% CI) | AUC (95% CI) | ||||||||
| 95 | 3.0 % | Adult-onset (n = 336) | yes | yes | 0.82 (0.77–0.87) | 0.83 (0.78–0.87) | ND | 0.69 (0.63–0.75) | ND |
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |||||||
| 101 | 3% + biopsy eos. ≥ 22/m2 | Adult symptomatic (n = 67) | no | yes | 0.79 (NR) | 0.71 (NR) | 0.84 (NR) | 0.62 (NR) | ND |
| AUC (95% CI | AUC (95% CI | AUC (95% CI | AUC (95% CI | ||||||
| 102 | 3% | Adult with loss of disease control (n = 47) | yes | yes | 0.76 (0.65-0.810.65−0.81) | 0.92 (0.85−0.97) | 0.56 (0.46−0.66) | 0.51 (0.41−0.61) | ND |
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | AUC (95% CI) |
Ref.#= reference number; eos = eosinophils; AUC = Area Under the Curve; CI = confidence interval; ICS = inhaled corticosteroid treatment; ND = not done, NR = not reported.
Inhaled corticosteroid therapy affects NO production by iNOS, reducing FeNO levels in treated asthmatic patients.104 For this reason, FeNO evaluation has been proposed to monitor disease control in asthmatic patients characterized by eosinophilic inflammatory phenotype at diagnosis. A 20% fall in FeNO or 10 ppb has been proposed as a significant response to inhaled corticosteroid treatment when starting values are >50 ppb or <50 ppb respectively.105 FeNO levels decrease two weeks after severe asthma exacerbations treated with systemic corticosteroids, and return to stable levels after 4–8 weeks.100 In patients with more than one exacerbation/year, treatment guided according to FeNO levels seems useful in maintaining disease stability.106 A systematic review and meta-analysis on sputum and FeNO guided treatment in asthmatic subjects confirmed the reduction of exacerbations in patients monitored with these biomarkers but disease control and lung function were similar to those patients followed in a traditional way.107 The same was found in patients with mild/moderate persistent asthma.108 FeNO proved useful in predicting response to ICS in patients with non-specific respiratory symptoms and lack of bronchodilator reversibility.109 In stable asthma, basal and serial evaluation of FeNO is not able to predict treatment failure confirming that this biomarker alone should not be recommended for this purpose.110
Recent results of a multicentre randomized control trial in patients with mild asthma showed that only in patients with blood eosinophil count greater than 0.3 × 109/L maintenance with budesonide plus as needed salbutamol was efficacious in decreasing exacerbations while FeNO or composite scores are not useful for this purpose.111
FeNO may help evaluate disease stability in pregnant asthmatic women, since during pregnancy, asthmatic patients monitored with FeNO reduced exacerbations and increased quality of life.112 Cough is a non-specific symptom of asthma in common with other conditions, sensitive to ICS treatment when caused by inflammation. FeNO has been proposed to monitor patients with corticosteroid responsive cough.113,114
FeNO as a predictor of target treatment response and effects of these therapies on FeNO levelsFeNO, together with other T2 biomarkers, has been used to monitor the efficacy of target therapies with monoclonal antibodies in severe asthmatic patients. Asthmatic patients with high FeNO (≥ 19.5 ppb) decrease the number of exacerbations when treated with omalizumab more than subjects with low FeNO (<19.5 ppb).115 Recent ERS/ATS task force for severe asthma management, concluded that FeNO, together with blood eosinophils, can identify which patients are more responsive to omalizumab in terms of reduction of exacerbations and impairment of lung functions, but more studies are needed to evaluate other outcomes.116 FeNO and blood periostin seem the best markers for selecting asthmatic patients to be treated with tralokinumab or with lebrikizumab, two anti IL-13 monoclonal antibodies.117,118 Treatment of severe asthmatic patients with dupilumab, an antibody to the α subunit of the IL-4 receptor, reduced disease exacerbations and FeNO levels, both in allergic and in non allergic patients.119 Furthermore, FeNO levels ≥ 25 ppb in severe asthmatic patients predict significant response to dupilumab.120
Not all target therapies aimed to inhibit T2 inflammation affect FeNO levels. FeNO is not reduced by anti-IL-5 monoclonal antibodies, which efficiently reduce eosinophilic inflammation but have limited effect on epithelial cell activation.121 Real world studies confirmed the efficacy of mepolizumab treatment without affecting FeNO levels.122
FeNO and blood eosinophils were not reduced by administration of fevipiprant, an oral prostaglandin D2 receptor 2 antagonist that was successful in reducing sputum eosinophils in patients with moderate/severe asthma and persistent high sputum eosinophils despite treatment with inhaled corticosteroids.123
Treatment targeting epithelial-cell–derived cytokine thymic stromal lymphopoietin (TSLP) resulted in a significant decrease of FeNO together with asthma exacerbations both in eosinophilic and in non-eosinophilic asthmatic subjects, suggesting the strict relationship between TSLP and FeNO production.124 Recently a mixed inflammatory pattern characterized by severe T2 and T17 high has been described. In this study, asthmatic patients with elevated just FeNO or with both elevated FeNO and blood eosinophils have increased IL-5, IL-8, IL-13 and IL-17A, which can cause a massive tissue destruction and consequently a more severe clinical phenotype,89 opening the possibility that FeNO can identify patients to target other molecules.
Table 3 summarises the effects of target therapies on FeNO and other biomarkers.
Variation of T2 biomarkers after target therapy in patients with severe asthma.
| Target therapy | MoAb | FeNO | Blood eosinophils | Serum periostin | Sputum eosinophils | Ref. # |
|---|---|---|---|---|---|---|
| Anti IgE | Omalizumab | ND | ↓ | ND | ↓ | 125 |
| ↓ | ↓ | ND | ↓ | 115 | ||
| Anti IL-5/IL-5R | Mepolizumab | = | ↓↓ | ND | ↓ | 121 |
| Reslizumab | ND | ↓ | ND | ↓ | 126 | |
| ↓ | ↓ | ND | ND | 127 | ||
| Benralizumab | ND | ↓↓ | ND | ↓↓ | 128 | |
| Anti IL-13 | Lebrikizumab | ↓ | = | ↓ | ND | 129 |
| Tralokinumab | ↓ | = | = | = | 117 | |
| Anti IL-4Rα | Dupilumab | ↓ | ↓ | ↓ | ND | 119 |
| Anti TSLP | Tezepelumab | ↓ | ↓ | ND | ↓ | 130 |
| Antagonist of prostaglandin D2 | Fevipiprant | = | = | ND | ↓ | 123 |
MoAb = Monoclonal Antibody; Ref.#= reference number; IL-= Interleukin-; IL-5R = Interleukin-5 Receptor; TSLP = Thymic stromal lymphopoietin; = unchanged, ND = not done.
Asthma and particularly severe asthma can benefit from rehabilitation programs.131 However, bronchial inflammation, at least type 2, does not seem to be decreased by breathing retraining programs, which instead have effects on asthma related quality of life.132 The same is true for high-intensity interval training performed by non-obese asthmatic patients which had no effect on airway inflammation evaluated with FeNO while a significant improvement in asthma control and quality of life was reported.133 FeNO also remained unchanged after 4 week therapist-led sessions to manage disease while asthma control increased.134 Aerobic training reduced FeNO and sputum eosinophils in asthmatic patients with higher airway inflammation at baseline.78
Clinical implicationsFeNO measurements determined changes in therapeutic programs in about 30% of the cases and in 90% when corticosteroid treatment was considered.135 Results of a real-world survey highlighted that airway inflammation is often underestimated by clinicians with respect to FeNO determination and its evaluation could be added to other measures to achieve asthma control.135 Expert committee of the Global Initiative for Asthma (GINA) document reported that having high FeNO is a risk factor for exacerbations in allergic asthmatics taking ICS. They also reported that, due to lack of long-term studies, FeNO cannot be recommended to step down ICS therapy, however FeNO guided treatment was recognized as evidence A in reducing exacerbations in children.1
Applicability to low income countriesAsthma is considered to be one of the chronic respiratory conditions, affecting the Disability-Adjusted Life Years index, which evaluates the years of life spent with disabilities.136 In many low income countries, prevention, diagnosis and management of asthma is quite difficult due to low perception of risk factors (indoor and outdoor exposures), low availability of diagnostic measures/devices, low adherence and use of alternative medicine, low possibility of follow-up,137,138 and difficulties in recovering high cost therapies for severe asthmatic patients. FeNO is an exhaled biomarker, which is easily detectable by on-line and off-line devices, frequently measured in children, in whom other inflammatory biomarkers have limited use. Measures can be easily performed by minimally trained personal, particularly for portable devices. This facilitates its spread in research and clinical settings, making the evaluation possible in many countries, including the less developed. FeNO was used to characterize severity of East African asthmatics, highlighting the reduced access to therapy in this area of the world.139 In rural zones of South Africa FeNO measurements have been used to assess airway inflammation in women exposed to pesticides.140 FeNO and sensitization state were evaluated in severe asthmatic children living at high altitude in Bogotá.141 Exposure to paraffin used for cooking in low socio-economic communities in Africa, was associated with increased FeNO levels and rhinitis symptoms in children.142 FeNO levels evaluated in Malaysian office workers were associated with atopy and respiratory symptoms and with the amount of sieved dust even when analysis was corrected for indoor temperature or relative air humidity.143,144
Published studies conducted in low income countries showed that FeNO evaluation might be useful in different settings to monitor asthmatic patients and to evaluate impact of different exposures on airway inflammation. Cost effectiveness studies on FeNO used both at diagnosis and during asthma monitoring in different countries,145–147 showed that FeNO can reduce the cost of disease management particularly in severe patients. This economic evaluation could also favour FeNO use in low-income countries, but more studies in this setting, particularly focused on costs and outcomes, are needed.
Research prioritiesAs proposed by GINA panel of experts, long follow-up studies focused on step-down ICS therapy based on low FeNO levels are needed.1 Furthermore, considering the utility of this biomarker to define T2 inflammation, large cohort studies in low income countries might reveal its usefulness in asthma prevention and disease control.
ConclusionsFeNO production by iNOS in asthma can vary and be associated with different disease markers. Many factors and interventional approaches can influence FeNO levels and should be considered in clinical settings and in follow-up studies in order to correctly interpret FeNO levels. Additional biomarkers have been recognized as T2 sign marks, and due to the complexity of the immunological pathways, they are only partially overlapping. ICS therapy significantly affects FeNO levels but target therapies with monoclonal antibodies impact on FeNO levels only when the target molecule interplays with mechanisms involved in FeNO production. FeNO should be considered a useful aid in low income countries to monitor asthmatic patients and to survey their exposure to possible inflammatory agents.
Authors’ contributionsPP, DV, SL, AGM, JWCA and AS conceived and drafted the manuscript with the support of GBM. AGM prepared figures. All authors critically reviewed and edited the final version prior to submission.
Conflicts of interestThe authors have no conflicts of interest to declare.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AGM was funded by Marie Skłodowska-Curie Actions [grant agreement no. 713660—PRONKJEWAIL—H2020-MSCA-COFUND-2015] outside the submitted work.