Duchenne Muscular Dystrophy (DMD) is the most common inherited muscle disease diagnosed in children, with a prevalence ranging between 1.3 and 2.1 per 10,000 live male births. Caused by a mutation of the dystrophin encoding gene located at Xp21, the disease results in a relentless progression of muscle weakness and wasting of the skeletal and cardiac muscle cells. Even though implementing nocturnal and daytime long-term ventilation and cough assistance has reduced the risk of respiratory complications, Acute Respiratory Failure (ARF) is still a common occurrence in DMD patients and a leading cause of death in the very advanced stages of the disease. The pathogenesis of ARF has been attributed to an imbalance between increased respiratory load and reduced diaphragmatic capacity. Well-known aetiologies include pneumonia, otherwise benign upper respiratory tract infection, and congestive heart failure.1
Acute colonic pseudo-obstruction (ACPO) is a rare disorder characterized by acute dilatation of part or all of the colon/rectum in the absence of any intrinsic or extrinsic mechanical obstruction. First described by Sir William Ogilvie in 1948, ACPO usually presents in hospitalized or institutionalized patients suffering from severe comorbid conditions, such as musculoskeletal abnormalities, trauma, surgery, or sepsis.2 Patients affected with the disorder typically present worsening abdominal distension and some degree of abdominal discomfort/pain and constipation.3 Abdominal overdistension can affect respiratory mechanics and blood gas exchange by causing a cranial shift of the diaphragm, reducing chest wall compliance and lung volume.4
DMD patients frequently present with intestinal motility disorders that have been attributed to immobility and weakness of the abdominal wall muscles. Altered dystrophin expression in the intestinal smooth muscle can also contribute to impaired gastrointestinal motility. As episodes of colonic pseudo-obstruction have been described in DMD patients,5 we have hypothesized that ACPO could have some bearing on the onset of acute respiratory decompensation.
For this reason, we collected and reviewed all of the medical records of the DMD patients with ARF admitted to our 4-bed Respiratory Intensive Care Unit (RICU) between January 1, 2005 and August 31, 2019 after informed consent release forms were obtained. At the time of admission to the RICU, the clinical and physiological parameters of these patients were consistent with ARF; in particular, each presented at least one of the following: (1) difficulty in breathing; (2) hypoxemia and/or hypercapnia; (3) acute respiratory acidosis. The causes of ARF were determined taking into consideration the patients’ clinical, radiological, hemodynamic, and laboratory test results. ACPO was diagnosed depending on the presence of abdominal distention and pain, nausea with vomiting, and/or constipation; the diagnosis was confirmed by radiographic evidence of colonic dilation >9cm and the exclusion of any mechanical obstructions.3
48 DMD patients with a primary diagnosis of ARF were admitted to our RICU during the study period. ACPO was the cause of ARF in 2 of the patients (4.2%). The causes of ARF in the other patients were: pneumonia (16 cases); upper respiratory tract infection (12 cases); acute congestive heart failure (10 cases); gastroparesis/malnutrition (3 cases); pneumothorax (2 cases); tracheo-innominate fistula (2 cases); abuse of sedatives (1 case).
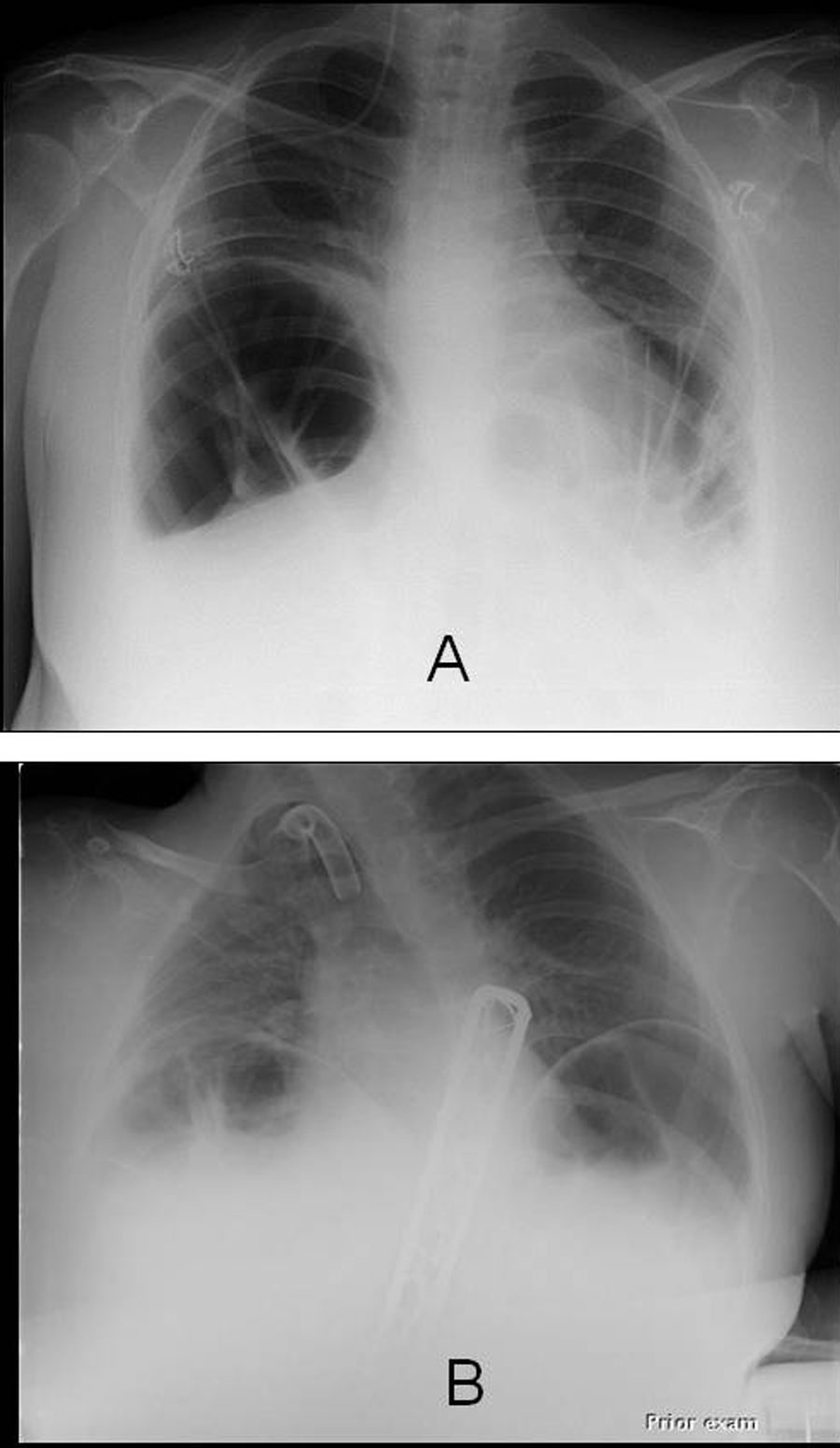
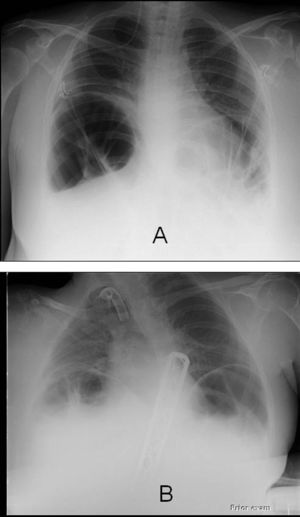
The baseline demographic, clinical and pulmonary function data, and the clinical and laboratory data at the time of their admission to the RICU of the 2 patients who were diagnosed with ACPO are outlined in Table 1; of note, patient 1 had been receiving home Non-Invasive Ventilation (NIV) administered by a pressure-limited ventilator, set on the assist/control mode, with Pressure Control (PC) at 18cmH2O, Respiratory Rate (RR) at 15breaths/min, Inspiratory Time at 1.4s, and PEEP at 4cmH2O. Both patients were receiving full-time ventilatory support on admission. In both cases of ACPO, distended bowel loops and marked diaphragmatic elevation were noted on an anteroposterior chest X-ray; parenchymal infiltrate, pleural effusion and/or pneumothorax were absent (Fig. 1).
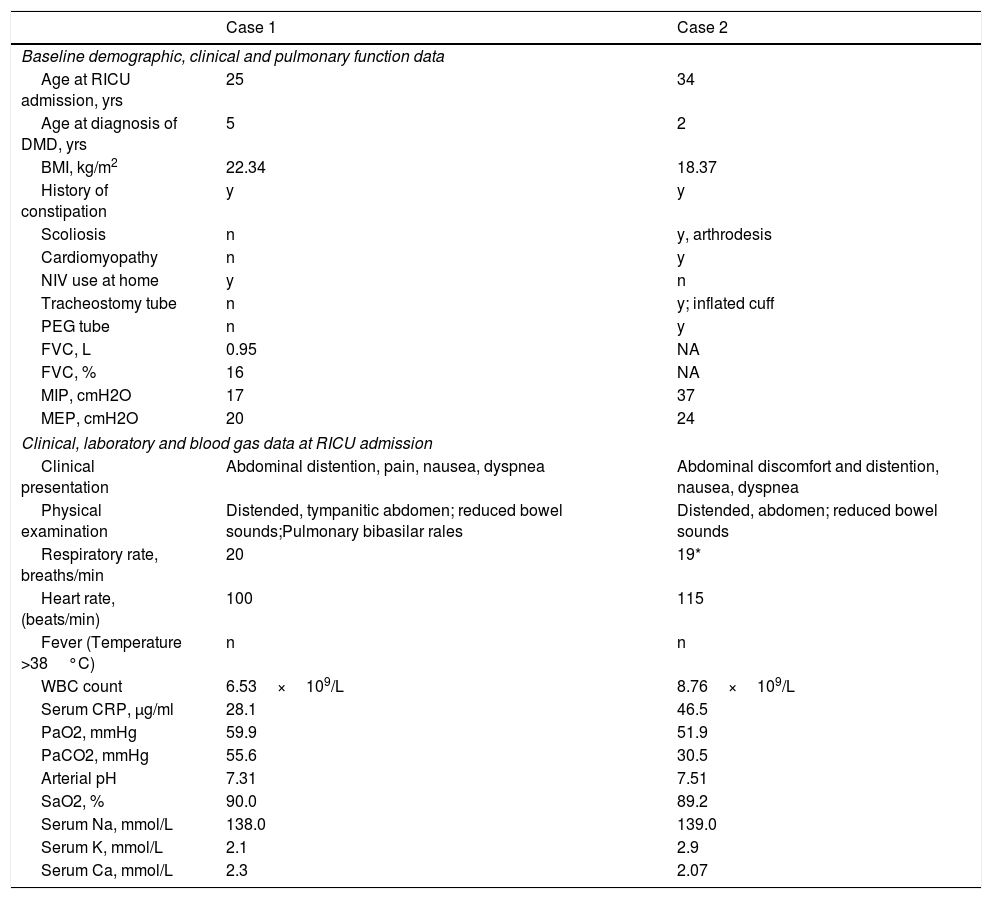
Baseline demographic, clinical and pulmonary function data, and data at Respiratory Intensive Care Unit admission of the 2 patients who were diagnosed with ACPO. Blood gas analysis was performed during assisted ventilation (ACPO=Acute Colonic Pseudo-Obstruction; BMI=Body Mass Index; CRP=C-Reactive Protein; FVC=Forced Vital Capacity; MIP=Maximum Inspiratory Pressure; MEP=Maximum Expiratory Pressure; NIV=Non-Invasive Ventilation; PEG=Percutaneous Gastrostomy).
| Case 1 | Case 2 | |
|---|---|---|
| Baseline demographic, clinical and pulmonary function data | ||
| Age at RICU admission, yrs | 25 | 34 |
| Age at diagnosis of DMD, yrs | 5 | 2 |
| BMI, kg/m2 | 22.34 | 18.37 |
| History of constipation | y | y |
| Scoliosis | n | y, arthrodesis |
| Cardiomyopathy | n | y |
| NIV use at home | y | n |
| Tracheostomy tube | n | y; inflated cuff |
| PEG tube | n | y |
| FVC, L | 0.95 | NA |
| FVC, % | 16 | NA |
| MIP, cmH2O | 17 | 37 |
| MEP, cmH2O | 20 | 24 |
| Clinical, laboratory and blood gas data at RICU admission | ||
| Clinical presentation | Abdominal distention, pain, nausea, dyspnea | Abdominal discomfort and distention, nausea, dyspnea |
| Physical examination | Distended, tympanitic abdomen; reduced bowel sounds;Pulmonary bibasilar rales | Distended, abdomen; reduced bowel sounds |
| Respiratory rate, breaths/min | 20 | 19* |
| Heart rate, (beats/min) | 100 | 115 |
| Fever (Temperature >38°C) | n | n |
| WBC count | 6.53×109/L | 8.76×109/L |
| Serum CRP, μg/ml | 28.1 | 46.5 |
| PaO2, mmHg | 59.9 | 51.9 |
| PaCO2, mmHg | 55.6 | 30.5 |
| Arterial pH | 7.31 | 7.51 |
| SaO2, % | 90.0 | 89.2 |
| Serum Na, mmol/L | 138.0 | 139.0 |
| Serum K, mmol/L | 2.1 | 2.9 |
| Serum Ca, mmol/L | 2.3 | 2.07 |
Both patients were prescribed the standard treatment for ACPO consisting of: nothing by mouth, a nasogastric tube (in case 1), postural changes, i.v. fluids, and electrolyte replacement. In case 2, PEG tube was used to remove swallowed air from stomach. In addition, a polyethylene glycol (PEG) 3350 was administered via nasogastric or PEG tube, a broad-spectrum antibiotic was prescribed, and a rectal catheter was inserted. Neostigmine was excluded to avoid the risk of bronchoconstriction and/or increased bronchial secretions. In case 1, the ventilator setting was readjusted to a reduced insufflational pressure of 10cmH2O. As patient n. 1 did not respond to supportive treatment, endoscopic decompression of the colon was successfully carried out. Both patients showed progressive clinical improvement leading to recovery of spontaneous breathing during daytime. Time to recovery was shorter for case 1 compared to case 2 (4 and 10 days after admission, respectively).
Our findings demonstrated that the relevance of ACPO as a cause of ARF was similar to that of the other less frequent causes of acute respiratory decompensation in DMD patients. The development of ARF could be explained, we hypothesize, by severe diaphragmatic weakness (demonstrated by the marked reduction in MIP in both cases) that could have reinforced the physiological response to increased intra-abdominal pressure, usually consisting of relaxation and ascent of the diaphragm permitting cephalic expansion of the abdominal cavity.6 Excessive diaphragmatic ascent could lead to a reduction in Respiratory System Compliance (Crs) shifting the lung volume to a lower portion of the volume–pressure curve. The result of reduced compliance would be increased work of breathing, potential fatigue of the respiratory muscles and, ultimately, acute respiratory decompensation.7 The application of NIV in one of the ACPO patients (n. 1) may have contributed to the development of ARF as the release of positive pressure by a non-invasive route can lead to esophageal insufflation and in turn to gastro-intestinal distension. In particular, we hypothesize that insufflational airway pressure around 20 cmH2O may have exceeded the lower esophageal sphincter pressure (approximately 20–25cm H2O, in a healthy adult).8 Abdominal overdistension in turn may have caused CO2 retention during NIV application, as delivered Tidal Volume decreases according to decreased Crs when the ventilator is set on a pressure-limited mode. In case 2, concomitant mild hypocalcemia may have facilitated impaired intestinal motility.
Worth noting, both of the patients presenting ACPO had a long-standing history of abdominal bloating and constipation. In the light of these findings, clinicians caring for DMD patients should be aware of the repercussions of intestinal dysmotility in these patients and prescribe appropriate measures to treat troublesome symptoms.
To conclude, although ACPO seems to be an infrequent cause of ARF, clinicians caring for DMD patients should be aware of the possibility of “pulmonary-colonic syndrome” and be prepared to identify and treat this potentially life-threatening condition precociously.
Conflicts of interestThe authors have no conflicts of interest to declare.