Lung recruitment of patients suffering from severe acute respiratory syndrome Corona-Virus 2 (SARS-CoV-2)-associated acute respiratory distress syndrome (ARDS) has been assessed functionally e.g. by electrical impedance tomography (EIT), determining compliance and right-to-left-shunt fraction or calculating the recruitment-to-inflation ratio,1 methods all of which demonstrated a varying degree of functional recruitability. By comparing the CT images obtained at two different airway pressures one can visualize recruitable areas in the lungs of patients with ARDS anatomically.2 Significant lung recruitability determined in this manner usually predicts a beneficial effect of positive end-expiratory pressure (PEEP) on oxygenation, relates inversely to the severity of lung injury and has prognostic implications.2 Here, we amend such anatomic recruitment data from dynamic chest CTs of two increasingly invasively ventilated male patients (59- and 67-years of age) in the early phase of severe SARS-CoV-2-associated ARDS.
Following a first chest CT under myorelaxation at a lower pressure (10 cmH2O) and a subsequent recruitment maneuver (PEEP 15 cmH2O; inspiratory pressure 35 cmH2O for one minute, then PEEP 25 cmH2O and inspiratory pressure 45 cmH2O for another minute) a second chest CT was performed with a 45 cmH2O inspiratory hold. CT image processing, lung segmentation and quantitative analysis were performed with custom-designed software (Maluna®).3 For each 0.0025 ml (2.5 mm³) voxel we determined the density in Hounsfield units (HU) from −1000 to +100, subdivided the density range into quantiles of five HU (giving 220 segments) and calculated the corresponding volume of each segment. Each voxel was classified as non- (−100 to +100 HU), poorly- (–500 to –101 HU), normally- (–900 to –501 HU), or over-aerated (–1000 to –901 HU).2 Overall recruitment potential was defined as the total change in the percentage of lung volume (in liters) classified as non-aerated between the two pressures. Lung weight was estimated as follows: Lung-volume (ml) × (mean density [HU] + 1000 [HU])/1000. Written informed consent for publication was obtained for both patients.
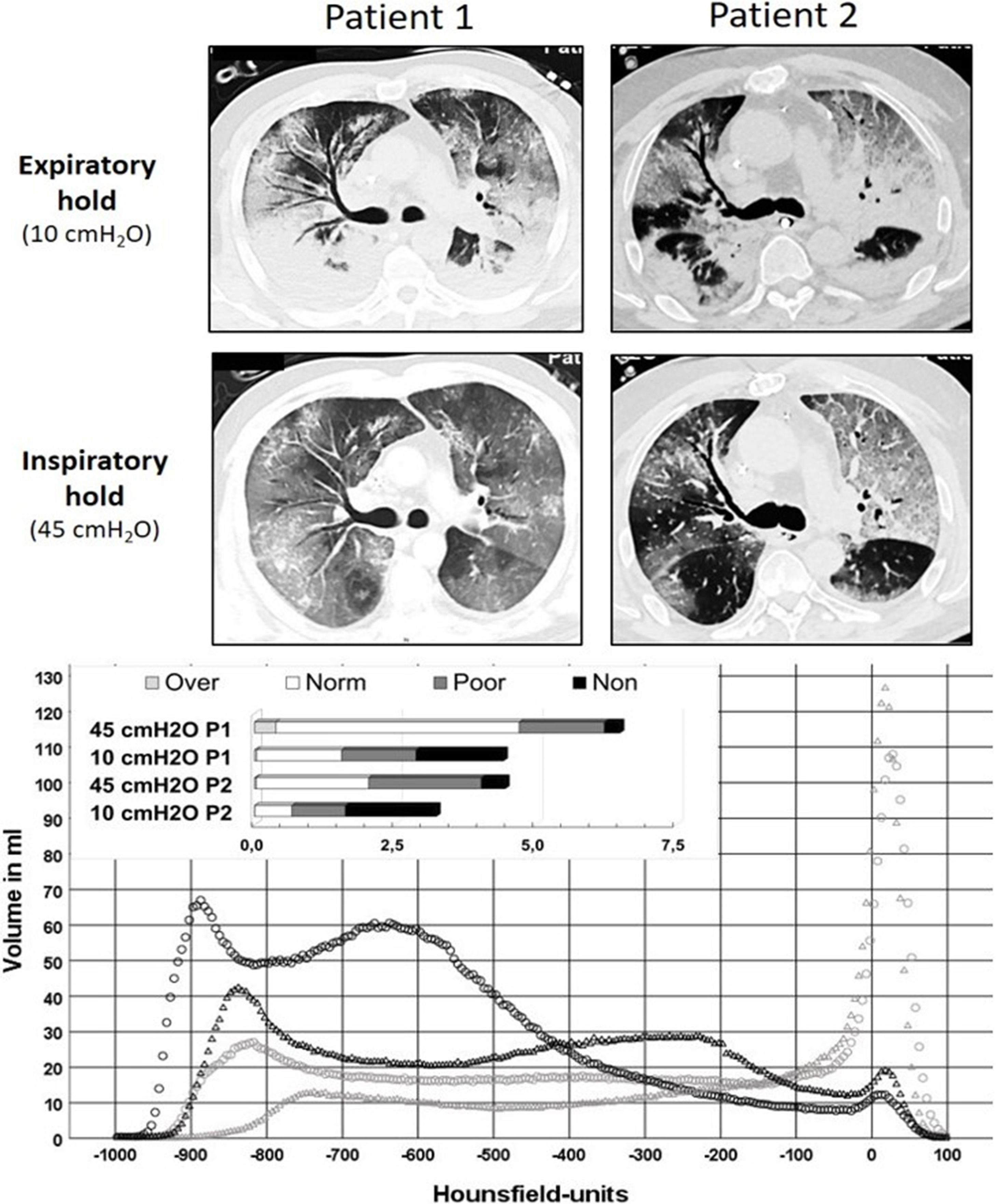
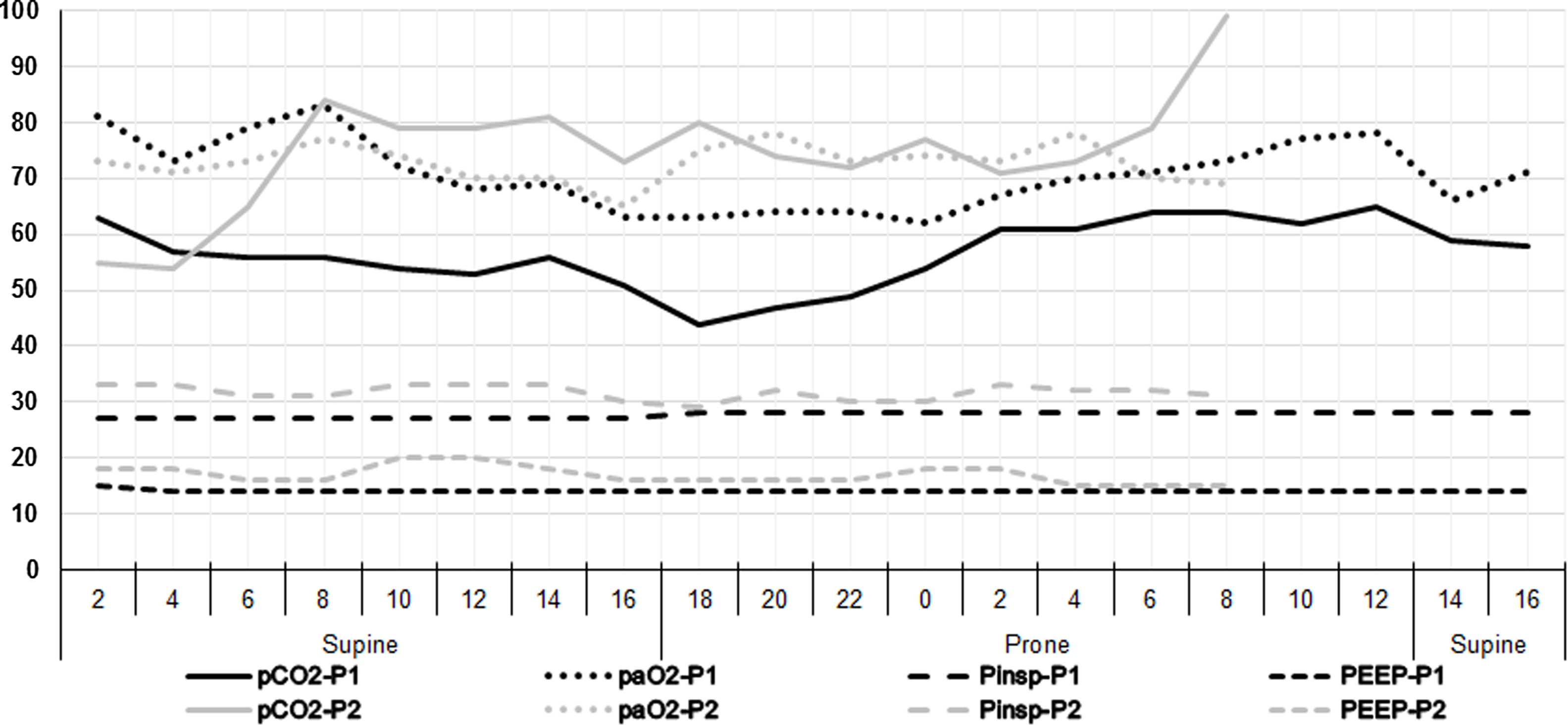
Chest CTs at 10 cmH2O showed a radiological picture compatible with severe viral pneumonia with multifocal, bilateral, subpleural, and peribronchovascular pure ground-glass opacities, air bronchogram, traction bronchiectasis as well as subtotal consolidations in the dorsal lower lobes (Fig. 1). The latter nearly disappeared with an inspiratory pressure of 45 cmH2O, while the percentage of non-aerated lung decreased from 35% and 36% (10 cmH2O) to 4% and 13% (45 cmH2O) for patients 1 and 2, respectively (Fig. 1). Likewise, the overall mean densities decreased from −375 ± 17 HU (standard error of the mean (SEM)) and −259 ± 20 HU (10 cmH2O) to −620 ± 9 HU and −488 ± 13 HU (45 cmH2O) while calculated lung weights amounted to nearly 3000 and 2500 g in patients 1 and 2, respectively. However, neither a fairly high PEEP (15 cmH2O — guided by continuous transpulmonary pressure measurements) nor rotational therapy were able to bring about relevant near-term change in the paO2/FiO2 ratio, which remained almost constantly below 100 (Fig. 2).
Upper part: sections from low-dose, native chest CTs of both patients during expiratory hold with PEEP 10 cmH2O, as well as during inspiratory hold (airway pressure 45 cmH2O). Although both patients exhibited a distinct recruitability of the lungs, the underlying COVID-19 pattern explains the poor functional effect of higher PEEP levels in improving oxygenation. Lower part: this graph displays the volumes determined in the chest CTs (y-axis) per a range of 5 Hounsfield units (x-axis) at 10 cmH2O (light grey) and 45 cmH2O (black). Circles represent data from patient 1, triangles data from patient 2. The insert at the upper left shows the absolute lung volumes in liters classified as non-aerated, poorly aerated, normally aerated and overaerated again comparing the two pressures in both patients. Note the differences between the absolute lung volumes at both pressure levels (at 45 cmH2O about 6.5 l for patient 1, about 4.5 l for patient 2).
Blood gases and concurrent ventilator settings during a two-day course. As both patients had a nearly identical time course regarding recruitment CT and begin of prone positioning, the data for both are shown in the same diagram. The x-axis shows two-hour time intervals approximatively corresponding to the times of the blood gas analyses. In addition, the x-axis indicates how the patient was positioned (supine or prone). The numbers on the y-axis refer to both the arterial oxygen (paO2) and carbon dioxide (pCO2) partial pressures in mmHg, as well as to the positive end-expiratory (PEEP) and the inspiratory (Pinsp) pressures in cmH2O. The inspiratory oxygen fraction (FiO2) was kept between 75 and 80% for most of the time period of the diagram. The data sets of patient 2 (light grey) end at 8 am on day 2 as extracorporeal membrane oxygenation was initiated at that time.
The observed clearing of dorsal consolidations under high PEEP during CT is well in line with the common recommendation to apply a hypoxemia-driven high PEEP, which should theoretically induce a pressure-dependent fluid transfer from the bronchioli and alveoli into the surrounding parenchyma,4 but also brings about possible detrimental effects like barotrauma, impairment of hemodynamics, and retention of carbon dioxide. Likewise, prone positioning is a well-established treatment option in severe ARDS and reduces the risk of gravity-dependent alveolar collapse and inflammatory pulmonary edema. However, contradicting the anatomic recruitment potential neither higher PEEP nor prone positioning significantly improved our patients’ condition in the short-term.2 This finding could relate to the severe and dynamic lung edema that is partially refractory to high ventilatory pressures or gravitational forces, and whose radiologic expression is the weak effect of high pressure on transparency in the CT in areas in which ground-glass opacities predominated. Accordingly, calculated lung weights were particularly high.2 Additionally, high extents of lung microvascular thrombosis and dysfunction causing a severe imbalance between ventilation and perfusion are increasingly reported in COVID-19-associated ARDS.5 In line with our observation, recent evidence indeed supports the application of low PEEP especially in the early stages of severe COVID-19-associated ARDS.6 On the other hand, prone positioning currently is generally recommended for treatment of patients with severe COVID-19-associated ARDS.6,7 However, its efficacy may be reduced in most severe forms of the disease.
In conclusion, anatomic recruitment of lung parenchyma was well preserved in two patients in the early phase of severe COVID-19-associated ARDS while functional recruitment was not. Severe hypoxemia, primarily good compliance, and increased lung weight may be typical features of the early phase of severe COVID-19-associated ARDS and these patients may therefore benefit more from a higher FiO2, a lower PEEP, and strict attention to fluid balance.
Conflicts of interestNone.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.