Besides pneumonia, the most frequent serious manifestation of COVID-19, another prominent manifestation is venous thromboembolism (VTE).1 Data on the prevalence/incidence of pulmonary embolism (PE) in COVID-19 are still limited and often focused on patients in intensive care units (ICU).2–4 Moreover, few data are available on the severity of PE and on the severity of parenchymal involvement in those who develop PE. Here, we present our data in 157 consecutive COVID-19 patients, hospitalized at Bichat Hospital, who underwent CT pulmonary angiography (CTPA) for suspected PE.
From March 6 to April 20 2020, 1097 patients were hospitalized for COVID-19 at Bichat Hospital, one of the reference centers for infectious diseases in Paris. Of the 157 patients who had CTPA, all with positive SARS-CoV-2 PCR results, 30 were hospitalized in the ICU, with 23 (76.7%) requiring invasive mechanical ventilation. The remaining 127 patients were hospitalized in the general ward for serious COVID-19 manifestations but without critical illness.
For patients hospitalized for COVID-19, the general policy at our institution is to recommend use of anticoagulant therapy based on low-molecular-weight heparin or unfractionated heparin, the intensity varying from low-prophylactic doses to curative treatment via high-prophylactic doses according to the severity of the respiratory condition, the presence of obesity or other risks factors of VTE. In all cases, CTPA was performed to exclude the responsibility of superimposed PE in patients hospitalized for severe COVID-19 and presenting stagnation or worsening of their respiratory condition. CT-angiography scans were acquired with 64-row or greater scanners after injection of 70–90mL of contrast material with a high concentration of iodine. Imaging was performed with use of a bolus-tracking technique and a threshold of 200 HU in the main pulmonary artery. Images were reconstructed with a slice thickness of 1mm in mediastinal and parenchymal windows. We retrospectively analyzed the CTPA results as well as the clinical and biological data for the patients. The quality of the examinations was sufficient to assess the presence or absence of PE up to the segmental level. One reader (AK, with 25 years of experience) classified PE location as proximal, lobar, or segmental. The study received approval from the ethics committee of the French Language Society of Pulmonology “Comité d’évaluation des protocoles de recherche observationnels (CEPRO)”.
The incidence of PE detected by CTPA in the 157 patients was 27% (8 of 30) in ICU patients and 12% (15 of 127) in ward patients. PE was proximal, lobar, segmental, and bilateral in 0%, 12.5%, 87.5%, and 25% and 7%, 33%, 60%, and 47% of cases in the ICU and general ward, respectively. CTPA revealed a concomitant evidence of worsening of pneumonia in 75% and 67% of ICU and ward patients with PE, respectively. When using an already published CT severity index (range 0–25),5 the CT score was 14 on the initial CT and 18 [14–19] at the time of PE (p=0.0883). Interestingly, the CT severity index of patients with and without PE showed no significant difference (18 [14–19] versus 14.5 [10–20]; p=0.2561).
Clinical and biological data are in Table 1.
Characteristics of COVID-19 patients with and without pulmonary embolism (PE) hospitalized in the intensive care unit (ICU) or general ward (GW).
| Variable | Total (n=157) | With PE (n=23) | Without PE (n=134) | p |
|---|---|---|---|---|
| GW patients | 127 (80.9%) | 15 (65.2%) | 112 (83.6%) | |
| ICU patients | 30 (19.1%) | 8 (34.8%) | 22 (16.4%) | |
| Clinical characteristics | ||||
| Age (years) | 63 [52–74] | 59 [52–79] | 63 [52–73.25] | 0.9185 |
| BMI (kg/m3) | 27.08 [24.5–30.73] | 28.11 [24.52–33.57] | 26.83 [24.5–30.65] | 0.5455 |
| History of VTE | 12 (7.6%) | 1 (4.3%) | 11 (8.2%) | 0.4912 |
| Diabetes | 48 (30.6%) | 6 (26.1%) | 42 (31.3%) | 0.6132 |
| Dyslipidemia | 37 (23.6%) | 6 (26.1%) | 31 (23.1%) | 0.7579 |
| Arterial hypertension | 78 (49.7%) | 11 (47.8%) | 67 (50%) | 0.8472 |
| Active malignancy | 5 (3.2%) | 3 (13%) | 2 (1.5%) | 0.0036 |
| Smoking status | ||||
| Never smoker | 109 | 18 | 91 | 0.427 |
| Former smoker | 41 | 5 | 36 | |
| Active smoker | 7 | 0 | 7 | |
| Chronic cardiac insufficiency | 10 (6.4%) | 2 (8.7%) | 8 (6%) | 0.621 |
| Chronic respiratory insufficiency | 1 (0.6%) | 0 | 1 (0.7%) | 0.6777 |
| Anticoagulant therapy | ||||
| No anticoagulant | 9 (5.7%) | 2 (8.7%) | 7 (5.2%) | 0.1232 |
| Low prophylactic dose | 39 (24.8%) | 5 (21.7%) | 34 (25.4%) | |
| High prophylactic dose | 90 (57.3%) | 10 (43.5%) | 80 (59.7%) | |
| Curative dose | 19 (12.1%) | 6 (26.1%) | 13 (9.7%) | |
| Time between symptoms onset and CTPA (days) | 13 [10–18] | 16 [12–23] | 12 [9–18] | 0.0164 |
| Time between hospitalization and CTPA (days) | 5 [2–9.5] | 8 [4–15] | 5 [2–8] | 0.0134 |
| Radiological characteristics | ||||
| Parenchymal evaluation by comparison with the previous chest CT scan | ||||
| Worsening | 107 (68.1%) | 16 (69.6%) | 91 (67.9%) | 0.2420 |
| Stability | 29 (18.5%) | 2 (8.7%) | 27 (20.2%) | |
| Improvement | 5 (3.2%) | 2 (8.7%) | 3 (2.2%) | |
| NA (no previous CT available) | 16 (10.2%) | 3 (13%) | 13 (9.7%) | |
| Biological characteristicsa,b | ||||
| d-Dimers (ng/ml) | 1249 [566–3394] | 7638 [2179–27544] | 1064 [497.5–1767] | <0.0001 |
| GW | 1055 [491–1852] | 27544 [7448–76306] | 870 [478–1573] | <0.0001 |
| ICU | 2179 [1280–7638] | 3898 [1556–16117] | 1747 [1089–6981] | 0.3074 |
| Fibrinogen (g/l) | 5.17 [4.325–11.81] | 4.94 [4.010–6.675] | 5.19 [4.34–6.21] | 0.9855 |
| GW | 5.15 [4.335–6.19] | 4.94 [3.045–6.41] | 5.19 [4.545–6.14] | 0.6511 |
| ICU | 5.23 [4.22–6.743] | 5.52 [4.46–7.043] | 5.23 [3.978–6.548] | 0.6047 |
| H-s troponin (μg/l) | 0.015 [0.015–0.032] | 0.015 [0.015–0.028] | 0.015 [0.015–0.032] | 0.44 |
| GW | 0.015 [0.015–0.0255] | 0.015 [0.015–0.0355] | 0.015 [0.015–0.026] | 0.7024 |
| ICU | 0.015 [0.015–0.06475] | 0.015 [0.015–0.03450] | 0.017 [0.015–0.1048] | 0.2341 |
| NT-proBNP (ng/l) | 324 [95–1335] | 253 [111.5–3096] | 329.5 [93.75–1307] | 0.7062 |
| GW | 244 [83–804.5] | 160 [82–520.5] | 307 [82–809] | 0.4165 |
| ICU | 1360 [392–3901] | 3096 [350.5–9674] | 1069 [392–3647] | 0.4815 |
| CRP (mg/l) | 52 [21–108.5] | 109.5 [31.25–181] | 47 [20.5–96.5] | 0.0292 |
| GW | 50 [21–96.5] | 117 [32.25–181] | 46 [20.5–93.5] | 0.0532 |
| ICU | 63.5 [21.25–189.8] | 99 [31.25–189.8] | 59.5 [17.25–191.3] | 0.5574 |
Data are presented as median [interquartile range] or number (percentage) where appropriate. Groups with and without PE were compared by Mann–Whitney U test or chi-square test, for quantitative and categorical variables, respectively. p<0.05 was defined as statistically significant.
Bold values signifies values are statistically significance.
Biological data from samples obtained within 48h before or after CT pulmonary angiography in the ICU and within 5 days before or after CT in the GW.
Missing data in ICU: d-dimers n=3, NT proBNP n=3, CRP n=2; missing data in GW: d-dimers n=59, fibrinogen n=33, troponin n=39, NT proBNP n=39, CRP n=6.
Abbreviations: PE: pulmonary embolism; GW: general ward, ICU: intensive care unit; BMI: body mass index; VTE: venous thromboembolism; CTPA: CT pulmonary angiography; H-s troponin: high sensitivity troponin; BNP: brain natriuretic peptide; CRP: C reactive protein.
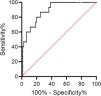
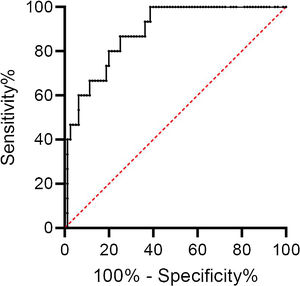
d-Dimers levels were significantly higher in ward patients with PE than those without; there was no difference in ICU patients. Using the age-adjusted d-dimers cut-off levels, the sensitivity, specificity, negative predictive value, and positive predictive value of d-dimers for the diagnosis of PE was 100%, 26%, 100%, and 21% respectively (Table 2). The ROC curve for d-dimer in hospitalized COVID patients with suspected PE is shown in Fig. 1.
Age-adjusted d-dimers for predicting pulmonary embolism in hospitalized COVID-19 patients with suspected pulmonary embolism: contingency table for sensitivity and specificity calculation.
| Age-adjusted d-dimers | Total (n=157) | PE (n=23) | No PE (n=134) |
|---|---|---|---|
| Positive | 73 | 15 | 58 |
| Negative | 20 | 0 | 20 |
| d-Dimers non available | 64 | 8 | 56 |
Data are presented as numbers.
Age-adjusted d-dimers cut-off level (ng/ml) was age multiplied by 10. d-Dimers were considered positive if they were above this threshold.
Abbreviation: PE: pulmonary embolism.
Overall, 94.3% of patients received anticoagulant therapy (at least low-prophylactic doses) at the time of CTPA (100% in ICU patients and 93% in ward patients). For patients with PE, the proportion receiving anticoagulant therapy (at least a low-prophylactic dose) at the time of diagnosis was 100% and 86.7% in the ICU and general ward, respectively.
There is mounting evidence that COVID-19 patients, particularly critically ill patients, are at increased risk of VTE.2–4,6,7 The underlying mechanism involves factors related to COVID-19, such as inflammatory state, hypercoagulability, and endothelial damage, along with classical risk factors of VTE (older age, obesity, dehydration, immobilization, mechanical ventilation) which are often present with severe COVID-19.
A high incidence of PE in COVID-19 patients hospitalized in ICUs has been already reported3,4,6,7 but data in non-ICU patients remain limited.6–9 Our data confirm the high incidence of PE in COVID-19 patients hospitalized in ICU but also indicate that PE is also frequently observed in COVID-19 patients hospitalized in the general ward, justifying a high degree of awareness by clinicians.
Not surprisingly, we found that COVID-19 is associated with an hyperinflammatory state that contributes to the hypercogulability and in turn to the risk of VTE but the levels of CRP and fibrinogen did not differ between those with and without PE. Overall, d-dimers levels were significantly higher in those with PE than in those without PE, in line with the results of other COVID-19 studies.2,6,8–10 However, d-dimers were not able to predict thrombotic events, especially in ICU patients.
COVID-19 patients hospitalized in general wards because of severe illness are at risk of worsened condition leading, in the most severe cases, to admission to an ICU because of critical symptoms (respiratory failure, shock, multiple organ dysfunction). Likewise, in the ICU, physicians caring for COVID-19 patients are used to facing a general deterioration, with respiratory symptoms at the forefront. In both cases, progression of pneumonia is most often responsible for the observed worsening of the respiratory condition but the latter could also be related to the occurrence of PE or to PE superimposed on extending pneumonia.
From a clinical point of view, the fact that, along with the diagnosis of PE, CTPA also demonstrated a worsening of pneumonia in the majority of cases is informative. Performing thoracic CT without angiography instead of CTPA would have led to missing a significant number of embolic events. With worsening or non-improvement in patients hospitalized for COVID-19, our results argue for (1) increased awareness by clinicians of the possible responsibility of superimposed PE, even though progression of pneumonia may also be documented on chest CT, and (2) use of CTPA, whenever possible. New diagnostic techniques of PE have been recently investigated.11 Given the high incidence of PE in hospitalized COVID patients, they could be useful in this setting.
Ethics approval and consent to participateThe study received approval from the ethics committee of the French Language Society of Pulmonology “Comité d’évaluation des protocoles de recherche observationnels (CEPRO)”.
Consent for publicationNot applicable.
Availability of data and materialAll data are available for reviewers on demand.
FundingNo funding.
Contributions of the authorsVB and HM wrote the manuscript.
GW provided assistance for statistical analysis.
XL, JFT, AD, BLJ, JG, SN, NA retrieved the clinical and biological data from the files.
AK and LS analyzed the CTPAs.
All the authors have checked the manuscript.
Conflict of interestDr. Mal reports grants from Pfizer, personal fees from Boehringer, non-financial support from Novartis, outside the submitted work. Dr. Timsit reports personal fees from Merck, personal fees from Pfizer, personal fees from Gilead, personal fees from Paratek, personal fees from Medimmune, outside the submitted work. The other authors have no conflicts of interest to declare.