Myelodysplastic syndromes (MDS) represent a group of myeloid haematopoietic malignant disorders at hight risk of transformation into acute myeloid leukaemia (AML).1 5-azacytidine is a deoxyribonucleic acid (DNA) methyltransferase inhibitor and cytotoxic drug used since the year 2000 for the treatment of adult patients with AML, blast counts 20-30% and multilineage dysplasia.2 The first application of this drug was in 1982, as a hypomethylating agent of the Y globin suppressor gene, to induce fetal haemoglobin in thalassemia.2 The most common side effects of Azacytidine are: weakness, nausea, vomiting, constipation, injection site reactions and insomnia.3 Although intersticial lung disease could be a classic complication of numerous therapeutics, it has been rarely described regarding 5-azacitidine.2 Adverse drug reactions (ADR), particularly those that can be appreciably harmful and life-threatening, must be described to predict hazards in future applications, resulting in adjustments to the dosage regimen or in its definitive withdrawal. The Naranjo scale is the most used metric to determine an ADR3 and its score can aid in identifying pulmonary toxicity. We also emphasise the importance of excluding the most common differential diagnosis, such as opportunist infections, diffuse alveolar haemorrhage (DAH), acute cardiogenic pulmonary oedema and leukaemic infiltration, detected in HRCT by the predilection of leukaemic cells involving the perilymphatic pulmonary interstitium4. After a literature review, we acknowledged this case as the 18th azacytidine-induced pneumonitis reported.
A 56-year-old male presented with pancytopenia, while admitted to the Pulmonology Department for the treatment of community acquired pneumonia complicated by pleural effusion. He was a former smoker (smoking load 40-unit-pack-year), had previous history of drug addiction (ended 15 years ago), namely cannabis, cocaine, and heroin, and a medical history of hypertension, type 2 diabetes, dyslipidaemia, insomnia, and major depression. He was chronically medicated with furosemide, glargine, atorvastatin/ezetimibe, sertraline, risperidone, and clonazepam. He underwent a thorough haematological evaluation that included medullar aspiration and a bone marrow biopsy. The results showed a MDS with progression to AML. Patient was discharged from the Pulmonology Department and admitted to the Ambulatory Haematological Service where he initiated treatment with 5-azacitidine at the conventional dosage of 75mg/m2 for 7 days.
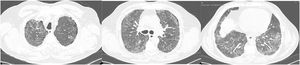
On the 5th day of the second therapeutic cycle (C2D5), patient complained of sudden dyspnea, fever (38.1°C) and presented with rapidly progressive hypoxemia (highest necessary FiO2 was 0.60). The chest X-Ray performed showed bilateral parenchymal infiltrates and blood analyses revealed a moderate increase of inflammatory parameters. Patient was admitted at the Hematology Department. A sepsis screen was carried out, empiric antibiotics prescribed, and an angio-thoracic CT was performed to exclude pulmonary thromboembolism (PE). The scan did not show PE but revealed diffuse parenchymal ground-glass densification with an “NSIP-like” pattern (Fig. 1).
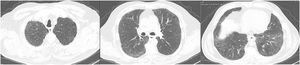
A pulmonology evaluation was requested. Suspected toxicity to azacytidine was suggested, once patient had a Naranjo score of 7 (probable ADR), and the drug was suspended. A complementary study was carried out. Bronchoalveolar lavage (BAL) showed 320,000 cels/mL, 93% lymphocytosis and an extensive negative microbiological study, namely for Pneumocystis jirovecii and CMV, considering the high risk for opportunistic pulmonary infections in the haemato-oncological setting. Respiratory function tests revealed slight restriction (TLC 74%) and severe decrease in single-breath carbon monoxide diffusing capacity of the lungs (DLCOSB 35% and KCO 61%). Despite the absence of elusive clinical features (no orthopnea, paroxysmal nocturnal dyspnea, crackles or peripheral oedema) and the NT-proBNP and HRCT pattern not suggesting cardiogenic pulmonary oedema, a heart evaluation is generally valuable and, therefore, an initial EKG and a subsequent echocardiogram were conducted and were both normal. The patient was prescribed 3 pulses of methylprednisolone 500 mg, followed by prednisolone 0.75 mg/kg in a slow tapering scheme, under PCP prophylaxis with trimethoprim/sulfamethoxazole. After 10 days there was clinical, gasometrical, and radiological improvement, having been discharged with ambulatory oxygen. After 3 weeks of treatment, a reassessment HRCT revealed a clear improvement, showing bilateral reduction of the ground-glass opacities (Fig. 2), and the 6MWT performed allowed the suspension of the previously prescribed oxygen.
In summary, azacytidine-induced pneumonitis is a diagnosis of exclusion and should be addressed after all relevant alternative diagnosis have been ruled out. This case confirmed an ILD secondary to toxicity to azacytidine due to the temporal link with the onset of the same, clinical-radiological agreement with previously described cases3, and the exclusion of differential diagnoses such as opportunistic infection, DAH, acute cardiogenic oedema (no clinical features or positive biomarkers for heart failure) or leukaemic infiltration (HRCT negative for suggestive features such as thickening of interlobular septa and bronchovascular bundles4). The expressive BAL lymphocytosis, suggesting an immune-mediated mechanism, and the clinical and radiological improvement after drug suspension and corticosteroid therapy institution, were also decisive supporting elements. In the appropriate clinical setting, this case report evokes the importance of a careful consideration towards the possibility of lung toxicity following hemato-oncological therapies, avoiding an unnecessary delay in drug suspension and the misuse of antibiotics.