Pulmonary rehabilitation (PR) is a fundamental intervention to manage COPD, however, maintaining its benefits is challenging. Engaging in physical activity might help to prolong PR benefits. This study assessed the efficacy and effectiveness of a personalised community-based physical activity programme to sustain physical activity and other health-related PR benefits, in people with COPD.
Materials and methodsThis was a multicentre, assessor blinded, randomised controlled trial. Following 12-weeks of PR, people with COPD were assigned to a six-months personalised community-based physical activity programme (experimental group), or to standard care (control group). Physical activity was assessed via: time spent in moderate to vigorous physical activities per day (primary outcome measure), steps/day and the brief physical activity assessment tool. Secondary outcomes included sedentary behaviour, functional status, peripheral muscle strength, balance, symptoms, emotional state, health-related quality of life, exacerbations and healthcare utilization. Assessments were performed immediately post-PR and after three- and six-months. Efficacy and effectiveness were evaluated using intention-to-treat and per-protocol analysis with linear mixed models.
ResultsSixty-one participants (experimental group: n = 32; control group: n = 29), with balanced baseline characteristics between groups (69.6 ± 8.5 years old, 84 % male, FEV1 57.1 ± 16.7 %predicted) were included. Changes in all physical activity outcomes and in one-minute sit-to-stand were significantly different (P < 0.05) between groups at the six-month follow-up. In the remaining outcomes there were no differences between groups.
ConclusionsThe community-based physical activity programme resulted in better physical activity levels and sit-to-stand performance, six-months after completing PR, in COPD. No additional benefits were observed for other secondary outcomes.
Pulmonary rehabilitation (PR) is a cost-effective intervention to manage people with chronic obstructive pulmonary disease (COPD), enhancing several health-related domains.1,2 A key aim of PR is to promote physical activity (PA),2 as PA can improve the COPD prognosis by decreasing the likelihood of exacerbations, hospitalizations and death.3-5
However, adopting and sustaining physically active behaviour after PR is highly challenging, and PR benefits are usually lost within 6 to 12 months after its completion.2,6 Effectiveness of post-PR maintenance programmes remains controversial, as study designs vary significantly, with systematic reviews reporting inconsistent findings.6-10 The optimal maintenance strategy capable of sustaining PR benefits is still unknown.
Engagement in regular long-lasting PA will most likely determine the maintenance of PR benefits.6,11 Nevertheless, to promote sustained physically active behaviour, interventions should be accessible and tailored to individual needs, goals and preferences, rather than assuming a “one size fits all” approach.10,11 Technology-based programmes, like those using social media, smartphone applications, websites or telemonitoring, have emerged as promising maintenance strategies to promote PA.10,12-14 However, their success still depends on patient digital literacy and access to technology, which remains a challenge for a considerable proportion of patients.15-17 Within the array of maintenance programme possibilities, both home-based and community-based approaches seem to be safe, feasible, and may provide different PA modalities that can be personalised to patient needs and preferences.18-21 Moreover, community-based maintenance programmes can also embrace several PA facilitators, such as: peer support, social connections, sense of inclusion within the community, supervision, enjoyment, accessibility and appropriate environmental conditions and infrastructures.19,22-27 In fact, lack of these elements in PA programmes has been suggested as reasons behind the nonadherence to a physically active lifestyle.5,19,24
Our study introduced an unprecedent programme by allowing people with COPD to select from various community-based PAs (e.g., senior or pool exercise classes) whichever best met their preferences and needs. The novelty of our study is, therefore, to unravel the role of these community resources to personalise PA and, ultimately, optimise outcomes in COPD.
We aimed to assess the efficacy and effectiveness of a six-month personalised community-based PA programme (PICk UP) on PA levels, six-months after completing PR, in people with COPD. As a secondary aim, effects on sedentary behaviour, functional exercise capacity and performance, peripheral muscle strength, balance, symptoms, emotional state, health-related quality of life, occurrence of exacerbations of COPD and healthcare usage were also evaluated. This study hypothesized that the PICk UP programme, targeting preferences and needs of participants with COPD, and based on an alliance among patients, health professionals and community, would be a solution to promote PA and prolong PR benefits.
Material and methodsStudy design and participantsPICk UP was a multicentre, parallel-group, assessor-blinded, randomised controlled trial. Ethical approval was granted from Centro Hospitalar do Baixo Vouga (Ref.15–05–2019), Unidade Investigação em Ciências da Saúde – Enfermagem (Ref.P620–10/2019), and Administração Regional de Saúde do Centro (Ref.16/2020). Informed consent was obtained from all participants and privacy was assured according to the European Union General Data Protection Regulation 2016/679 (GPDR). This study was registered on clinicaltrials.gov (NCT04223362) and follows the CONSORT statement28 and the Template for Intervention Description and Replication Checklist.29
Participants were recruited from outpatient community-based PR programmes30 (further details in the appendix) conducted in the Respiratory Research and Rehabilitation Laboratory (Lab3R), School of Health Sciences, University of Aveiro and in four primary health-care centres of Portugal (Aveiro, Estarreja, Oliveira do Bairro and Montemor-o-Velho), between February 2020 and July 2022. Recruitment was preceded by pneumologist, or general practitioner referral and participants were included if they: i) had a clinical diagnosis of COPD; ii) were clinically stable; ii) fulfilled the criteria to be managed in the community (appendix); and iii) completed the post-PR assessment. Individuals presenting signs of neoplasic/immunologic disease, or an unstable/significant cardiac, musculoskeletal, neuromuscular or psychiatric condition limiting the ability to exercise or co-operate were excluded.
Randomisation and blindingAfter the post-PR assessment, participants were randomised to the experimental group (EG: PICk UP programme) or to the control group (CG: standard care), using a 1:1 allocation ratio and random block sizes of two and four. An independent researcher used online software (https://www.sealedenvelope.com/simple-randomiser/v1/lists) to generate a computer-based random allocation list, stratified by location (Aveiro, Estarreja, Oliveira do Bairro and Montemor-o-Velho), and informed participants about their group allocation via phone call.
Assessors were not involved in the PICk UP programme provision and were blinded to group allocation and previous outcome measure results. Participants and data analysis responsible were not blinded to allocation, due to the nature of intervention and human resources constraints.
InterventionPICk up programmeParticipants within the EG enrolled in a six-month supervised personalised community-based PA programme, after completing an outpatient community-based PR programme.30,31 The physiotherapist that followed them during PR was responsible for the integration process. During the first month, participants could select among the available community-based PAs (senior exercise class, pool exercise class, gym or Chi Kung/Qigong), previously deemed as safe and of moderate intensity,32 whichever they wanted to try. The suitability of each PA modality was discussed with the physiotherapist to ensure it matched individual needs and minimized safety risks. Participants with more severe conditions (e.g., oxygen dependents or individuals with significant comorbidities) were also carefully discussed with the referring doctor. The community-based PAs were conducted in the facilities (sports centre, swimming pool or city council hall) of the four site locations. Whenever participants tried new community-based PAs, the physiotherapist that followed them during PR was present to ascertain safety. After this month, participants had to commit to one or two PAs, according to their preferences, and were recommended to attend those twice weekly for another five-months. The physiotherapist provided personalised support to participants (on-site supervision or phone-call) based on their needs, the PA characteristics, and his/her professional judgment during the intervention period. This support consisted of inquiring about adherence to the PAs, addressing any challenge/need that emerged, and encouraging participants to continue with the PA programme. The minimum support provided comprised one on-site supervision of each PA modality the participant decided to try (lasting approximately 1 h), and two phone-call after three- and six-months to check enrolment on the PA programme and schedule participant's assessment (lasting a maximum of 15 min each). Additional support (on-site or phone-call as deemed appropriate) was planned whenever: i) safety concerns emerged (e.g., oxygen desaturation or increased risk of falls); ii) participants’ self-efficacy was insufficient to proceed independently (e.g., difficulty in equipment handling); iii) participants requested additional support (e.g., perceived lack of confidence with the PA); iv) fitness instructor requested additional support (e.g., due to class size constraints the instructor could not provide proper feedback to the participant). The goal was to reduce support over time, ultimately aiming for independence from the physiotherapist. Throughout all community-based PAs, participants self-monitored intensity and were instructed by either fitness instructors and physiotherapists (during the integration phase) to exercise at their own pace while targeting moderate exertion (according to the training previously provided during PR, i.e., 3 to 6 on the modified Borg Scale). Participants received a diary to record their adherence to the PICk UP programme and/or other types of PAs (e.g., walking), and the occurrence of any adverse events. During the six-months trial, any concerns regarding the participants’ condition raised by the fitness instructor or the physiotherapist were promptly discussed with the referring doctor. The appendix includes further details regarding the PICk UP programme.
Standard careParticipants within the CG received the same outpatient community-based PR programme as the EG30,31 (further details in the appendix). No changes to their daily routine were provided during the trial period.
OutcomesThe primary outcome measure, collected immediately after and at three- and six-months post-PR, was time spent in moderate to vigorous PAs (MVPA) per day, assessed using accelerometery.33 Participants wore an ActiGraph GT3X+, placed above the right waist, for seven consecutive days for 24 h (removal was advised only during water activities). ActiGraph data was analyzed with ActiLife v6.10.4 (ActiGraph, Pensacola, FL, USA). To comply with wear-time validation participants had to have used the ActiGraph for at least four days during eight hours (from 7am to 10pm on both weekdays or weekends).34 ActiGraph processing criteria are detailed in the appendix.
Initially, the PICk UP primary outcome measure was steps/day. In February 2021, the primary outcome measure was changed to MVPA/day, to capture PA more comprehensively since community-based PAs are not based only on walking activities. Although participants recruitment began in February 2020, the trial was suspended shortly after due to the COVID-19 outbreak. When recruitment restarted, in April 2021, the primary outcome measure had already been changed, and a new sample size had been computed.
Secondary PA outcome measures included: MVPA/week, steps/day, time spent in sedentary behaviour/day/week (accelerometery); brief physical activity assessment tool (BPAAT); six-minute walk test (6MWT); 1-minute sit-to-stand test (1-minSTS); 28-item physical performance test; quadriceps maximal voluntary contraction and handgrip (dynamometry); Brief-Balance Evaluation Systems Test; COPD Assessment Test; Hospital Anxiety and Depression Scale; Checklist of Individual Strength-fatigue subscale; Functional Assessment of Chronic Illness therapy–fatigue subscale; St. George's Respiratory Questionnaire (SGRQ); and occurrence of exacerbations of COPD, hospitalizations and visits to the emergency department. In the EG, occurrence of adverse events and adherence to the community-based PAs were collected using the diary registries. All outcome measures were collected post-PR and after three- and six-months, and results were shared with the referring doctor. Each data collection moment lasted a maximum of 90 min.
Statistical analysisA sample size calculation was computed using G*Power3.1.9.6 for the within-between interaction of a mixed ANOVA with two groups (EG/CG) and 3 measurements (immediately after, and three- and six-months post-PR) of the primary outcome (MVPA/day). The sample size for MVPA/day was estimated considering an eta squared (η2) of 0.21 (Cohen's f = 0.516), obtained from a study using a PA-focused behavioural intervention during and after three-months of PR in COPD.35 A smaller f value of 0.30 was chosen to account for a longer follow-up period and differences in selected time-points. Considering: α=0.05, power=0.80, repeated measures correlation=0.5, nonsphericity correction=1, and expected effect size f = 0.30, the required sample size was 20 participants. As the anticipated dropout rate was 50 %,36,37 the final anticipated sample size was 40 participants (20/group).
We evaluated the effectiveness of PICk UP using an intention-to-treat (ITT) analysis and efficacy using a per-protocol analysis. The ITT analysis included all randomised participants. The per-protocol analysis included only participants adhering to the proposed intervention: i) completed both the post-PR and the six-months assessments (for accelerometer outcomes, only participants who complied with the wear time validation criteria were considered); ii) within the EG, those who enrolled in the PICk UP programme with at least 50 % attendance; iii) within the CG, those not enrolling in community-based PAs. Linear mixed models (LMM), or generalised linear mixed models (GLMM), including timepoint, group allocation and their interaction as fixed effects, and participants codes as random intercepts were used. A statistically significant interaction effect, time*group, indicated that the groups behaved differently over time. Post-hoc multiple comparisons were performed. For both ITT and per-protocol sets, two types of analysis were conducted: i) unadjusted; and ii) adjusted, including as covariates variables which were different between groups at baseline. Exclusively for the purpose of defining which covariates should be included in the adjusted analyses, differences between groups at baseline were explored with independent Student's t-test, U-Mann-Whitney test or Fisher's exact test, as appropriate. Furthermore, for the per-protocol analysis, variables associated with the likelihood of adhering to the proposed intervention were also included as covariates.38 The appendix includes more details regarding the LMM/GLMM.
Group differences on proportion of participants reporting an exacerbation, hospitalization or emergency department visit at the six-month follow-up were analysed using the ꭓ2 test.
Descriptive analyses were performed for the whole sample, by group allocation and by site location. Quantitative variables were summarized using mean±standard deviation or median [interquartile range], based on their distribution (Shapiro-Wilk test). Categorical variables were presented as counts (percentages). A P < 0.05 was considered statistically significant. Statistical analyses were conducted using RStudio Version 2022.12.0 + 353 running R version 4.2.0. Plots were created using GraphPad Prism 6.
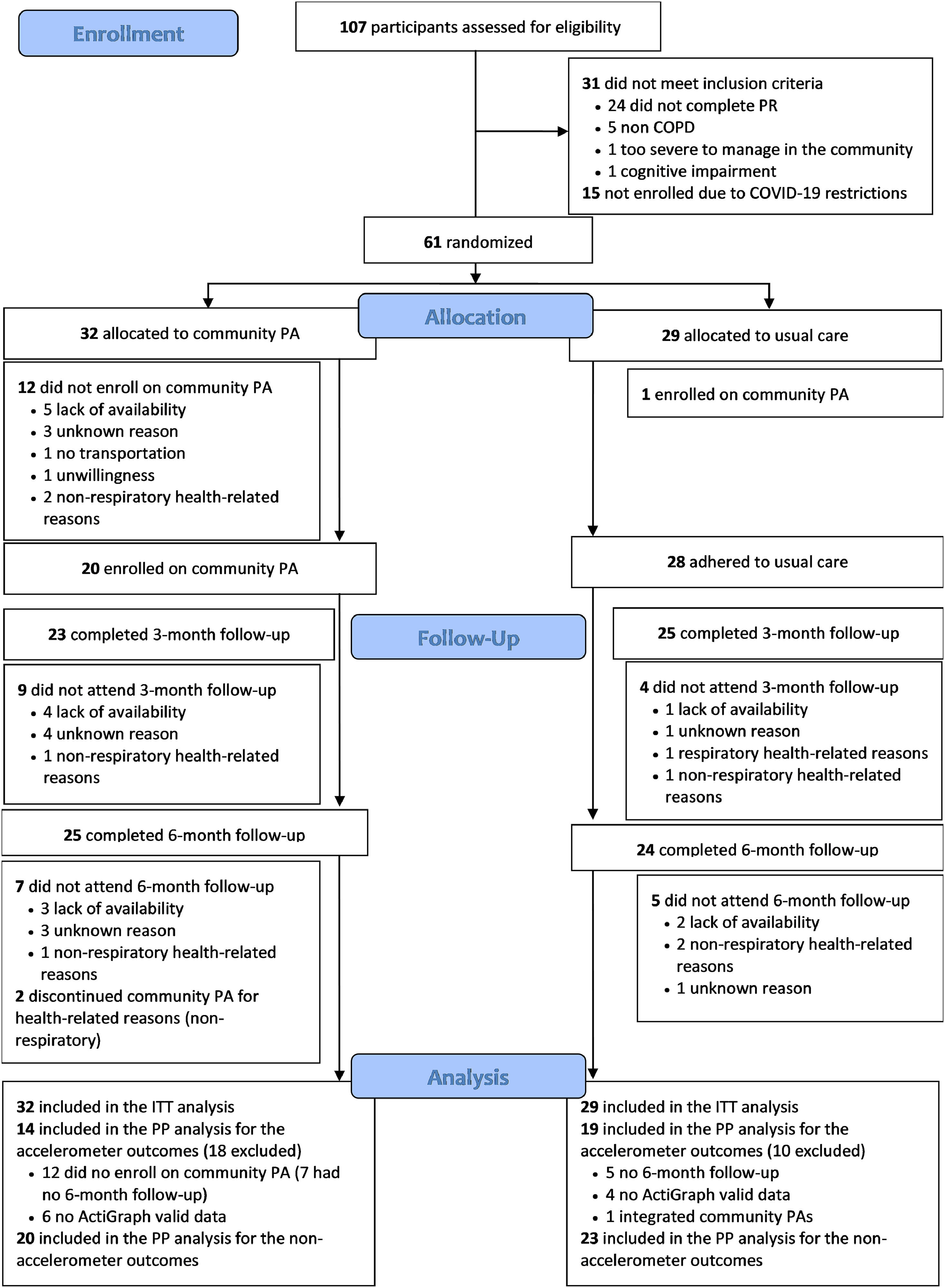
ResultsAmong the 107 participants assessed for eligibility (between February 2020 and July 2022), 61 fulfilled the inclusion criteria and were randomised, 32 to the EG and 29 to the CG. Number of participants randomised per site location were as follows: 13 in Aveiro, 27 in Estarreja, 13 in Oliveira do Bairro and 8 in Montemor-o-Velho. Characteristics of participants among sites were similar, except for differences in the BPAAT within the ITT set (P = 0.044) (Tables A1 and A2 from appendix). Overall, 49 (80 %) individuals with COPD completed the six-month follow-up (EG:25; CG:24; between January 2022 and January 2023). The per-protocol analysis included 33 participants (EG:14; CG:19) for the accelerometer outcomes and 43 (EG:20; CG:23) for the remaining outcomes. The CONSORT flowchart is shown in Fig. 1.
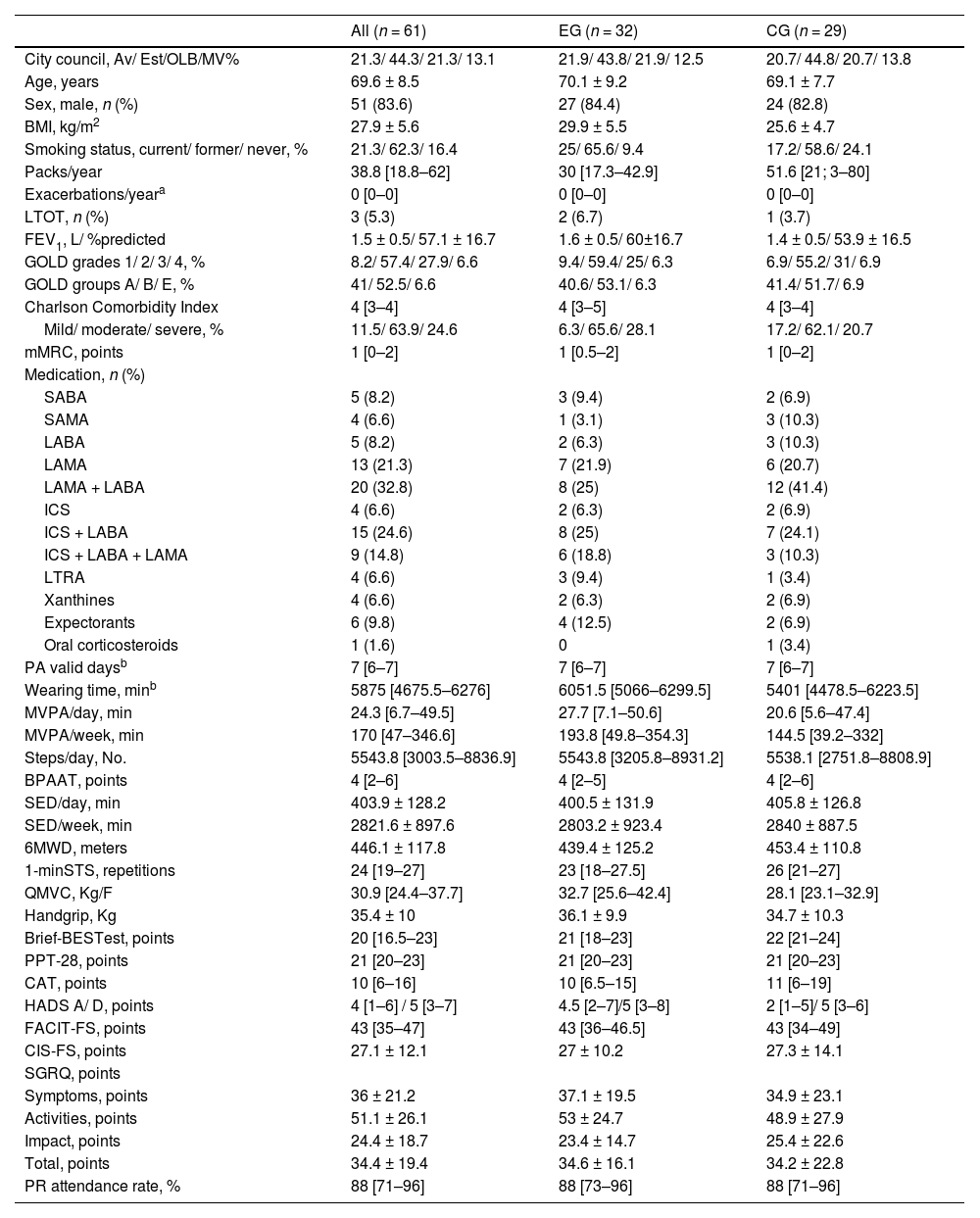
Baseline characteristics (post-PR) for the ITT (Table 1), per-protocol (Table A3 from appendix) sets were balanced among groups except for pack-years (greater in the CG) and body mass index (greater in the EG). Thus, these variables were included as covariates in ITT and per-protocol adjusted models. In the subset of the accelerometer data analysis, participants adherent to the proposed intervention presented higher PR attendance rates compared to non-adherent ones (Table A4 from appendix). Therefore, PR attendance rate was also included as a covariate in the adjusted model for the accelerometer data per-protocol analysis.
Baseline characteristics of participants with chronic obstructive pulmonary disease included in the intention-to-treat analysis of the PICk UP trial.
| All (n = 61) | EG (n = 32) | CG (n = 29) | |
|---|---|---|---|
| City council, Av/ Est/OLB/MV% | 21.3/ 44.3/ 21.3/ 13.1 | 21.9/ 43.8/ 21.9/ 12.5 | 20.7/ 44.8/ 20.7/ 13.8 |
| Age, years | 69.6 ± 8.5 | 70.1 ± 9.2 | 69.1 ± 7.7 |
| Sex, male, n (%) | 51 (83.6) | 27 (84.4) | 24 (82.8) |
| BMI, kg/m2 | 27.9 ± 5.6 | 29.9 ± 5.5 | 25.6 ± 4.7 |
| Smoking status, current/ former/ never, % | 21.3/ 62.3/ 16.4 | 25/ 65.6/ 9.4 | 17.2/ 58.6/ 24.1 |
| Packs/year | 38.8 [18.8–62] | 30 [17.3–42.9] | 51.6 [21; 3–80] |
| Exacerbations/yeara | 0 [0–0] | 0 [0–0] | 0 [0–0] |
| LTOT, n (%) | 3 (5.3) | 2 (6.7) | 1 (3.7) |
| FEV1, L/ %predicted | 1.5 ± 0.5/ 57.1 ± 16.7 | 1.6 ± 0.5/ 60±16.7 | 1.4 ± 0.5/ 53.9 ± 16.5 |
| GOLD grades 1/ 2/ 3/ 4, % | 8.2/ 57.4/ 27.9/ 6.6 | 9.4/ 59.4/ 25/ 6.3 | 6.9/ 55.2/ 31/ 6.9 |
| GOLD groups A/ B/ E, % | 41/ 52.5/ 6.6 | 40.6/ 53.1/ 6.3 | 41.4/ 51.7/ 6.9 |
| Charlson Comorbidity Index | 4 [3–4] | 4 [3–5] | 4 [3–4] |
| Mild/ moderate/ severe, % | 11.5/ 63.9/ 24.6 | 6.3/ 65.6/ 28.1 | 17.2/ 62.1/ 20.7 |
| mMRC, points | 1 [0–2] | 1 [0.5–2] | 1 [0–2] |
| Medication, n (%) | |||
| SABA | 5 (8.2) | 3 (9.4) | 2 (6.9) |
| SAMA | 4 (6.6) | 1 (3.1) | 3 (10.3) |
| LABA | 5 (8.2) | 2 (6.3) | 3 (10.3) |
| LAMA | 13 (21.3) | 7 (21.9) | 6 (20.7) |
| LAMA + LABA | 20 (32.8) | 8 (25) | 12 (41.4) |
| ICS | 4 (6.6) | 2 (6.3) | 2 (6.9) |
| ICS + LABA | 15 (24.6) | 8 (25) | 7 (24.1) |
| ICS + LABA + LAMA | 9 (14.8) | 6 (18.8) | 3 (10.3) |
| LTRA | 4 (6.6) | 3 (9.4) | 1 (3.4) |
| Xanthines | 4 (6.6) | 2 (6.3) | 2 (6.9) |
| Expectorants | 6 (9.8) | 4 (12.5) | 2 (6.9) |
| Oral corticosteroids | 1 (1.6) | 0 | 1 (3.4) |
| PA valid daysb | 7 [6–7] | 7 [6–7] | 7 [6–7] |
| Wearing time, minb | 5875 [4675.5–6276] | 6051.5 [5066–6299.5] | 5401 [4478.5–6223.5] |
| MVPA/day, min | 24.3 [6.7–49.5] | 27.7 [7.1–50.6] | 20.6 [5.6–47.4] |
| MVPA/week, min | 170 [47–346.6] | 193.8 [49.8–354.3] | 144.5 [39.2–332] |
| Steps/day, No. | 5543.8 [3003.5–8836.9] | 5543.8 [3205.8–8931.2] | 5538.1 [2751.8–8808.9] |
| BPAAT, points | 4 [2–6] | 4 [2–5] | 4 [2–6] |
| SED/day, min | 403.9 ± 128.2 | 400.5 ± 131.9 | 405.8 ± 126.8 |
| SED/week, min | 2821.6 ± 897.6 | 2803.2 ± 923.4 | 2840 ± 887.5 |
| 6MWD, meters | 446.1 ± 117.8 | 439.4 ± 125.2 | 453.4 ± 110.8 |
| 1-minSTS, repetitions | 24 [19–27] | 23 [18–27.5] | 26 [21–27] |
| QMVC, Kg/F | 30.9 [24.4–37.7] | 32.7 [25.6–42.4] | 28.1 [23.1–32.9] |
| Handgrip, Kg | 35.4 ± 10 | 36.1 ± 9.9 | 34.7 ± 10.3 |
| Brief-BESTest, points | 20 [16.5–23] | 21 [18–23] | 22 [21–24] |
| PPT-28, points | 21 [20–23] | 21 [20–23] | 21 [20–23] |
| CAT, points | 10 [6–16] | 10 [6.5–15] | 11 [6–19] |
| HADS A/ D, points | 4 [1–6] / 5 [3–7] | 4.5 [2–7]/5 [3–8] | 2 [1–5]/ 5 [3–6] |
| FACIT-FS, points | 43 [35–47] | 43 [36–46.5] | 43 [34–49] |
| CIS-FS, points | 27.1 ± 12.1 | 27 ± 10.2 | 27.3 ± 14.1 |
| SGRQ, points | |||
| Symptoms, points | 36 ± 21.2 | 37.1 ± 19.5 | 34.9 ± 23.1 |
| Activities, points | 51.1 ± 26.1 | 53 ± 24.7 | 48.9 ± 27.9 |
| Impact, points | 24.4 ± 18.7 | 23.4 ± 14.7 | 25.4 ± 22.6 |
| Total, points | 34.4 ± 19.4 | 34.6 ± 16.1 | 34.2 ± 22.8 |
| PR attendance rate, % | 88 [71–96] | 88 [73–96] | 88 [71–96] |
Values are presented as n (%), mean±standard deviation or median [interquartile range], unless otherwise stated. Missing data was present in the following variables: ActiGraph data (MVPA/day, MVPA/week, steps/day, SED/day and SED/week), n = 56 (EG=28; CG=28); 1-minSTS, n = 59 (EG=32; CG=27); Brief-BESTest, n = 60 (EG=31; CG=29).
Only participants complying with the ActiGraph wear time validation criteria were included, n = 56 (EG=28; CG=28).
Legend: Av: Aveiro; BMI: body mass index; BPAAT: Brief physical activity assessment tool; Brief-BESTest: Brief-Balance Evaluation Systems Test; CAT: COPD assessment test; CIS-FS: Checklist Individual Strength-fatigue subscale; CG: control group; COPD: chronic obstructive pulmonary disease; EG: experimental group; EST: Estarreja; FACIT-FS: Functional Assessment of Chronic Illness therapy–fatigue subscale; FEV1: forced expiratory volume in one second; GOLD: Global Initiative for chronic obstructive lung disease; HADS-A: Hospital Anxiety and Depression scale (A - anxiety, D – depression); ICS: inhaled corticosteroid; LABA: long-acting beta-agonists; LAMA: long-acting muscarinic antagonist; LRTA: leukotriene receptor antagonist; LTOT: long-term oxygen therapy; min: minutes; mMRC: modified Medical Research Council questionnaire; MV: Montemor-o-Velho; MVPA: time spent in moderate to vigorous physical activity; OLB: Oliveira-do-Bairro; PA: physical activity; No.: number; PPT-28: 28-item physical performance test; PR: pulmonary rehabilitation; QMVC: quadriceps maximal voluntary contraction; SABA: short-acting beta-agonists; SAMA: short-acting muscarinic antagonist; SED: time spent in sedentary behaviour; SGRQ: St. George's Respiratory Questionnaire; 1-minSTS: 1-minute sit-to-stand test; 6MWD: 6-minute walk distance.
Of the 20 participants from the EG that enrolled in the community-based PAs (14[7–51] days between randomisation and PA integration), six (30 %) tried more than one type of community-based PAs. Specifically, 11 tried senior exercise classes, nine tried gym, six tried pool exercise classes and two tried Chi Kung. Following the initial experimental month, seven (35 %) enrolled in gym, six (30 %) in senior exercise classes, three (15 %) in pool exercise classes, two (10 %) in Chi Kung and two (10 %) opted for a mix of pool exercise classes and gym. On average, community-based PAs consisted of 36[28–48] sessions throughout the six-month period. Physiotherapists supervised 1[1–4.3] PA session and contacted participants via phone call 2[2–3] times, thus, spending approximately 6 h with each participant of the EG (4:30 h collecting data, 1 h supervising the integration in the community-based PA and 30 min scheduling assessments and providing support).
Except for sedentary behaviour, COPD assessment test, and the activities sub-scale of the SGRQ, PR improved significantly all the measured outcomes (Table A5 from appendix). Before enrolling in PR, participants presented low levels of PA, engaging only 15 min in MVPA/day and being mostly categorised as physically inactive (BPAAT of 0 [0–4] points) (Table A5 from appendix).
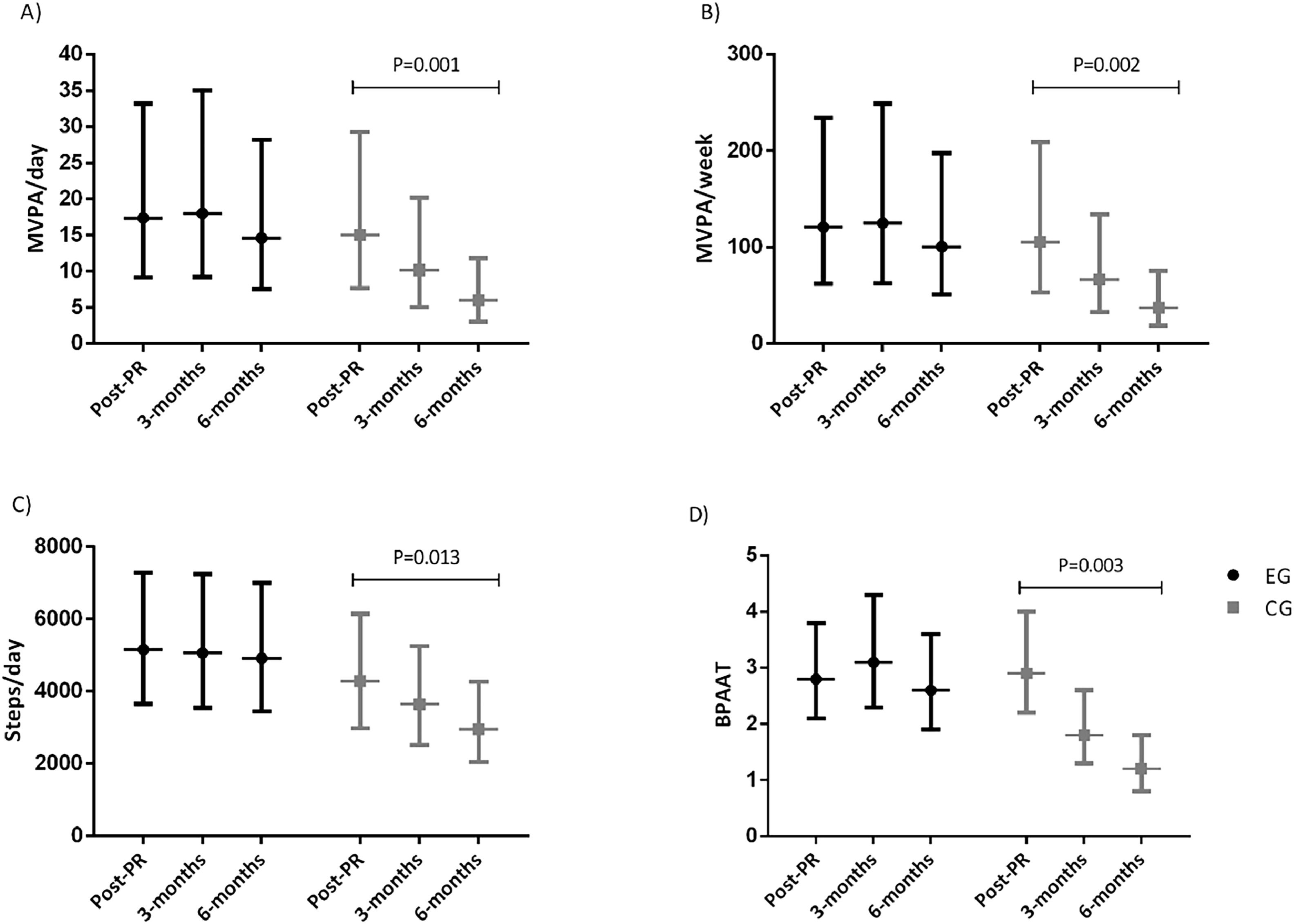
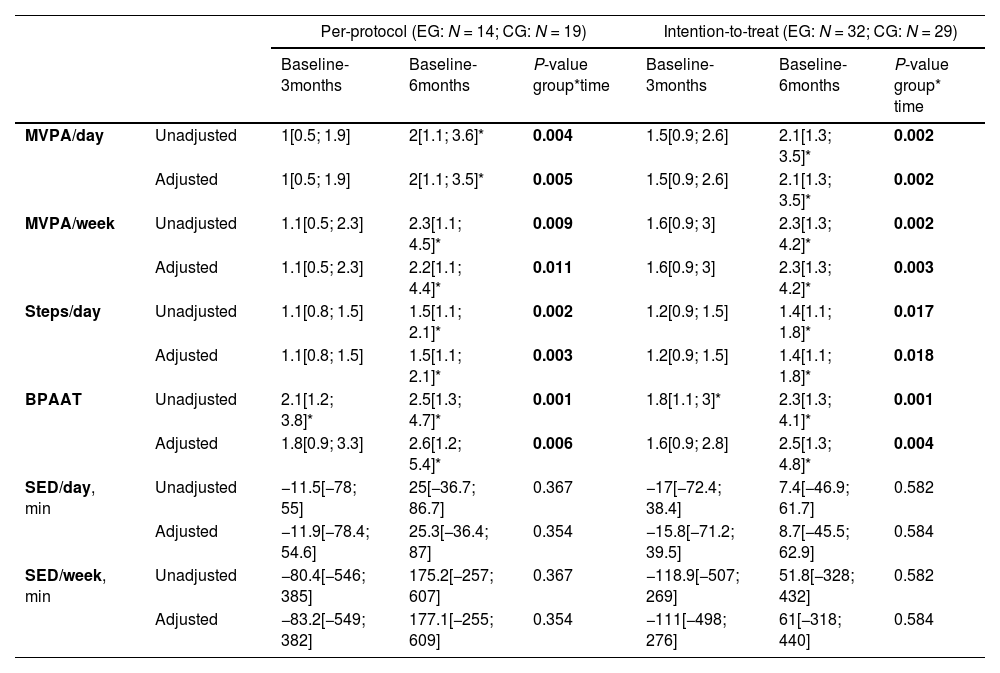
Physical activity and sedentary behaviour outcomesActiGraph valid days and wear-time data is provided in the appendix. Between group changes, at three- and six-month assessments, in the PA and sedentary behaviour outcomes, for the ITT and per-protocol analysis are presented in Table 2. The ITT and per-protocol analysis yielded similar PA and sedentary behaviour results. There was a significant time-group interaction for all PA but not for the sedentary behaviour outcomes. Between group changes in all PA outcomes were significantly different at the six-month follow-up (greater decline in the CG). According to the unadjusted BPAAT analysis, differences between group changes were also present at the three-month follow-up. Regarding within group changes, both in the ITT and per-protocol analyses all PA outcomes declined significantly in the CG from baseline to the 6-month, but no significant within-differences were observed in the EG (Fig. 2 and Table A6). In the ITT unadjusted analysis, in the CG, MVPA/day decreased from 15[8; 29] minutes (geometric mean [95 % confidence interval]) at the baseline to 6[3; 12] minutes at the six-month follow-up, and from 17[9; 33] to 15[8; 28] minutes in the EG. Similarly, in the CG, MVPA/week decreased from 106[53; 209] at the baseline to 37[19; 76] minutes at the six-month follow-up, and from 121[62; 234] to 101[5; 198] in the EG (Fig. 2 and Table A6). The decline observed on MVPA/day/week was 2.1 to 2.3 times greater in the CG compared to the EG (Table 2). For steps/day, the CG decreased from 4280[2980; 6147] at the baseline to 2952[2040; 4272] at the six-month follow-up, and from 5154[3651; 7275] to 4915[3452; 6997] in the EG (Fig. 2 and Table A6). The decline observed in steps/day was 1.4 times greater in the CG compared to the EG (Table 2). Unadjusted and adjusted means used in the LMM/GLMM models for PA and sedentary behaviour outcomes are presented in the appendix (Tables A9–12).
Between group changes for the physical activity and sedentary behaviour outcomes of the PICk UP trial.
| Per-protocol (EG: N = 14; CG: N = 19) | Intention-to-treat (EG: N = 32; CG: N = 29) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline-3months | Baseline-6months | P-value group*time | Baseline-3months | Baseline-6months | P-value group* time | ||
| MVPA/day | Unadjusted | 1[0.5; 1.9] | 2[1.1; 3.6]* | 0.004 | 1.5[0.9; 2.6] | 2.1[1.3; 3.5]* | 0.002 |
| Adjusted | 1[0.5; 1.9] | 2[1.1; 3.5]* | 0.005 | 1.5[0.9; 2.6] | 2.1[1.3; 3.5]* | 0.002 | |
| MVPA/week | Unadjusted | 1.1[0.5; 2.3] | 2.3[1.1; 4.5]* | 0.009 | 1.6[0.9; 3] | 2.3[1.3; 4.2]* | 0.002 |
| Adjusted | 1.1[0.5; 2.3] | 2.2[1.1; 4.4]* | 0.011 | 1.6[0.9; 3] | 2.3[1.3; 4.2]* | 0.003 | |
| Steps/day | Unadjusted | 1.1[0.8; 1.5] | 1.5[1.1; 2.1]* | 0.002 | 1.2[0.9; 1.5] | 1.4[1.1; 1.8]* | 0.017 |
| Adjusted | 1.1[0.8; 1.5] | 1.5[1.1; 2.1]* | 0.003 | 1.2[0.9; 1.5] | 1.4[1.1; 1.8]* | 0.018 | |
| BPAAT | Unadjusted | 2.1[1.2; 3.8]* | 2.5[1.3; 4.7]* | 0.001 | 1.8[1.1; 3]* | 2.3[1.3; 4.1]* | 0.001 |
| Adjusted | 1.8[0.9; 3.3] | 2.6[1.2; 5.4]* | 0.006 | 1.6[0.9; 2.8] | 2.5[1.3; 4.8]* | 0.004 | |
| SED/day, min | Unadjusted | −11.5[−78; 55] | 25[−36.7; 86.7] | 0.367 | −17[−72.4; 38.4] | 7.4[−46.9; 61.7] | 0.582 |
| Adjusted | −11.9[−78.4; 54.6] | 25.3[−36.4; 87] | 0.354 | −15.8[−71.2; 39.5] | 8.7[−45.5; 62.9] | 0.584 | |
| SED/week, min | Unadjusted | −80.4[−546; 385] | 175.2[−257; 607] | 0.367 | −118.9[−507; 269] | 51.8[−328; 432] | 0.582 |
| Adjusted | −83.2[−549; 382] | 177.1[−255; 609] | 0.354 | −111[−498; 276] | 61[−318; 440] | 0.584 | |
The BPAAT per-protocol data analysis included 43 participants (EG=20; CG=23). MVPA/day, MVPA/week, steps/day and BPAAT are presented as mean ratio [95 %CI]; mean ratios were considered significant if the 95 %CI excluded the value 1; ratios were calculated as: (CG baseline ÷ CG 3 months) ÷ (EG baseline ÷ EG 3 months); and (CG baseline ÷ CG 6 months) ÷ (EG baseline ÷ EG 6 months). SED/day and SED/week are presented as mean difference [95 %CI]; mean differences were considered significant if the 95 %CI excluded the value zero; differences were calculated as: (CG baseline – CG 3 months) – (EG baseline – EG 3 months); and (CG baseline – CG 6 months) – (EG baseline – EG 6 months).
P-value for the multiple comparison <0.05 (Sidak adjustment). Adjusted models included pack-years and body mass-index as factors. Additionally, adjusted models for the per-protocol analysis using accelerometer outcomes included pulmonary rehabilitation attendance rate as factor. A sensitivity analysis was conducted for the BPAAT, adding site location as a covariate in the intention-to-treat adjusted model, with no change observed in the results.
Legend: BPAAT: brief physical activity assessment tool; CG: control group; EG: experimental group; MVPA: time spent in moderate to vigorous physical activity; SED: time spent in sedentary behaviour per day.
Unadjusted geometric means and 95 % confidence intervals used in the linear-mixed models of the intention-to-treat analysis in A) time spent in moderate to vigorous physical activity per day; B) time spent in moderate to vigorous physical activity per week; C) steps/day; and D) Brief physical activity assessment tool of the PICk UP trial.
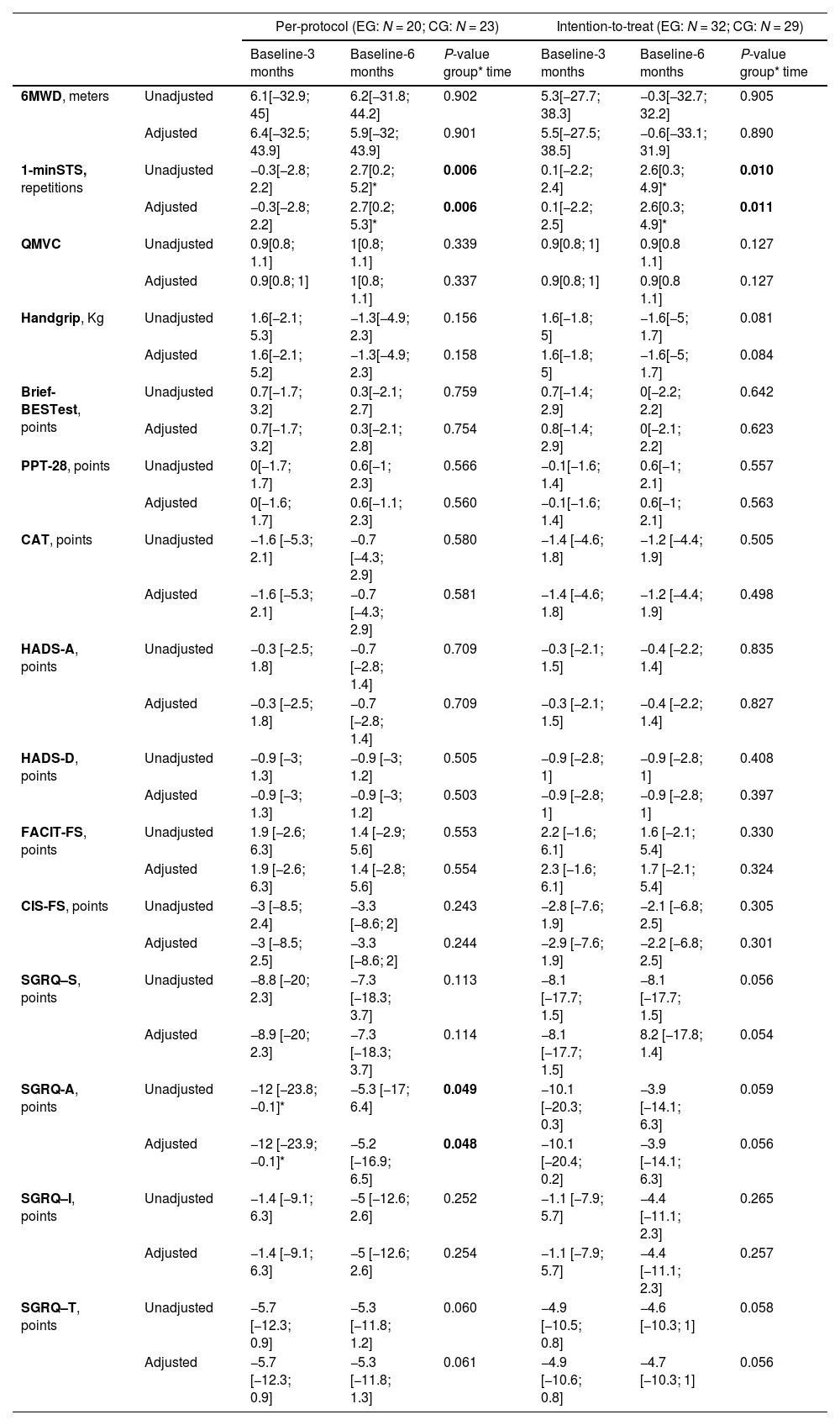
Between group changes, at three- and six-month assessments, in all other health-related outcome measures for the ITT and per-protocol analysis are presented in Table 3. In both analyses there was a significant time-group interaction for the 1-minSTS test. Differences between group changes in the 1-minSTS were significant at the six-month follow-up (greater decline in the CG). According to the ITT analysis, the CG performed 2.6 less repetitions at the six-month follow-up than at the baseline, compared to the EG. Additionally, in the per-protocol analysis, there was also a significant time-group interaction in the SGRQ activities’ subscale. For the remaining secondary outcome measures, no differences between group changes were observed. Regarding within group changes, in all analyses, in the CG, the 1-minSTS declined significantly from baseline to the 6-month, and, in the EG, the SGRQ activities subscale improved significantly from baseline to the 3-month follow-up (Table A7 and A8). Unadjusted and adjusted means used in the LMM/GLMM models are presented in the appendix (Tables A9–12).
Between group changes for other health-related outcome measures of the PICk UP trial.
| Per-protocol (EG: N = 20; CG: N = 23) | Intention-to-treat (EG: N = 32; CG: N = 29) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline-3 months | Baseline-6 months | P-value group* time | Baseline-3 months | Baseline-6 months | P-value group* time | ||
| 6MWD, meters | Unadjusted | 6.1[−32.9; 45] | 6.2[−31.8; 44.2] | 0.902 | 5.3[−27.7; 38.3] | −0.3[−32.7; 32.2] | 0.905 |
| Adjusted | 6.4[−32.5; 43.9] | 5.9[−32; 43.9] | 0.901 | 5.5[−27.5; 38.5] | −0.6[−33.1; 31.9] | 0.890 | |
| 1-minSTS, repetitions | Unadjusted | −0.3[−2.8; 2.2] | 2.7[0.2; 5.2]* | 0.006 | 0.1[−2.2; 2.4] | 2.6[0.3; 4.9]* | 0.010 |
| Adjusted | −0.3[−2.8; 2.2] | 2.7[0.2; 5.3]* | 0.006 | 0.1[−2.2; 2.5] | 2.6[0.3; 4.9]* | 0.011 | |
| QMVC | Unadjusted | 0.9[0.8; 1.1] | 1[0.8; 1.1] | 0.339 | 0.9[0.8; 1] | 0.9[0.8 1.1] | 0.127 |
| Adjusted | 0.9[0.8; 1] | 1[0.8; 1.1] | 0.337 | 0.9[0.8; 1] | 0.9[0.8 1.1] | 0.127 | |
| Handgrip, Kg | Unadjusted | 1.6[−2.1; 5.3] | −1.3[−4.9; 2.3] | 0.156 | 1.6[−1.8; 5] | −1.6[−5; 1.7] | 0.081 |
| Adjusted | 1.6[−2.1; 5.2] | −1.3[−4.9; 2.3] | 0.158 | 1.6[−1.8; 5] | −1.6[−5; 1.7] | 0.084 | |
| Brief-BESTest, points | Unadjusted | 0.7[−1.7; 3.2] | 0.3[−2.1; 2.7] | 0.759 | 0.7[−1.4; 2.9] | 0[−2.2; 2.2] | 0.642 |
| Adjusted | 0.7[−1.7; 3.2] | 0.3[−2.1; 2.8] | 0.754 | 0.8[−1.4; 2.9] | 0[−2.1; 2.2] | 0.623 | |
| PPT-28, points | Unadjusted | 0[−1.7; 1.7] | 0.6[−1; 2.3] | 0.566 | −0.1[−1.6; 1.4] | 0.6[−1; 2.1] | 0.557 |
| Adjusted | 0[−1.6; 1.7] | 0.6[−1.1; 2.3] | 0.560 | −0.1[−1.6; 1.4] | 0.6[−1; 2.1] | 0.563 | |
| CAT, points | Unadjusted | −1.6 [−5.3; 2.1] | −0.7 [−4.3; 2.9] | 0.580 | −1.4 [−4.6; 1.8] | −1.2 [−4.4; 1.9] | 0.505 |
| Adjusted | −1.6 [−5.3; 2.1] | −0.7 [−4.3; 2.9] | 0.581 | −1.4 [−4.6; 1.8] | −1.2 [−4.4; 1.9] | 0.498 | |
| HADS-A, points | Unadjusted | −0.3 [−2.5; 1.8] | −0.7 [−2.8; 1.4] | 0.709 | −0.3 [−2.1; 1.5] | −0.4 [−2.2; 1.4] | 0.835 |
| Adjusted | −0.3 [−2.5; 1.8] | −0.7 [−2.8; 1.4] | 0.709 | −0.3 [−2.1; 1.5] | −0.4 [−2.2; 1.4] | 0.827 | |
| HADS-D, points | Unadjusted | −0.9 [−3; 1.3] | −0.9 [−3; 1.2] | 0.505 | −0.9 [−2.8; 1] | −0.9 [−2.8; 1] | 0.408 |
| Adjusted | −0.9 [−3; 1.3] | −0.9 [−3; 1.2] | 0.503 | −0.9 [−2.8; 1] | −0.9 [−2.8; 1] | 0.397 | |
| FACIT-FS, points | Unadjusted | 1.9 [−2.6; 6.3] | 1.4 [−2.9; 5.6] | 0.553 | 2.2 [−1.6; 6.1] | 1.6 [−2.1; 5.4] | 0.330 |
| Adjusted | 1.9 [−2.6; 6.3] | 1.4 [−2.8; 5.6] | 0.554 | 2.3 [−1.6; 6.1] | 1.7 [−2.1; 5.4] | 0.324 | |
| CIS-FS, points | Unadjusted | −3 [−8.5; 2.4] | −3.3 [−8.6; 2] | 0.243 | −2.8 [−7.6; 1.9] | −2.1 [−6.8; 2.5] | 0.305 |
| Adjusted | −3 [−8.5; 2.5] | −3.3 [−8.6; 2] | 0.244 | −2.9 [−7.6; 1.9] | −2.2 [−6.8; 2.5] | 0.301 | |
| SGRQ–S, points | Unadjusted | −8.8 [−20; 2.3] | −7.3 [−18.3; 3.7] | 0.113 | −8.1 [−17.7; 1.5] | −8.1 [−17.7; 1.5] | 0.056 |
| Adjusted | −8.9 [−20; 2.3] | −7.3 [−18.3; 3.7] | 0.114 | −8.1 [−17.7; 1.5] | 8.2 [−17.8; 1.4] | 0.054 | |
| SGRQ-A, points | Unadjusted | −12 [−23.8;−0.1]* | −5.3 [−17; 6.4] | 0.049 | −10.1 [−20.3; 0.3] | −3.9 [−14.1; 6.3] | 0.059 |
| Adjusted | −12 [−23.9; −0.1]* | −5.2 [−16.9; 6.5] | 0.048 | −10.1 [−20.4; 0.2] | −3.9 [−14.1; 6.3] | 0.056 | |
| SGRQ–I, points | Unadjusted | −1.4 [−9.1; 6.3] | −5 [−12.6; 2.6] | 0.252 | −1.1 [−7.9; 5.7] | −4.4 [−11.1; 2.3] | 0.265 |
| Adjusted | −1.4 [−9.1; 6.3] | −5 [−12.6; 2.6] | 0.254 | −1.1 [−7.9; 5.7] | −4.4 [−11.1; 2.3] | 0.257 | |
| SGRQ–T, points | Unadjusted | −5.7 [−12.3; 0.9] | −5.3 [−11.8; 1.2] | 0.060 | −4.9 [−10.5; 0.8] | −4.6 [−10.3; 1] | 0.058 |
| Adjusted | −5.7 [−12.3; 0.9] | −5.3 [−11.8; 1.3] | 0.061 | −4.9 [−10.6; 0.8] | −4.7 [−10.3; 1] | 0.056 | |
QMVC data are presented as mean ratio [95 %CI]; ratios were calculated as: (CG baseline ÷ CG 3 months) ÷ (EG baseline ÷ EG 3 months); and (CG baseline ÷ CG 6 months) ÷ (EG baseline ÷ EG 6 months). The remaining outcomes are presented as mean difference [95 %CI]; mean differences were considered significant if the 95 %CI excluded the value zero; differences were calculated as: (CG baseline – CG 3 months) – (EG baseline – EG 3 months); and (CG baseline – CG 6 months) – (EG baseline – EG 6 months).
P-value for the multiple comparison <0.05 (Sidak adjustment). Adjusted models included pack-years and body mass-index as factors.
Legend: Brief-BESTest: Brief-Balance Evaluation Systems; CAT: COPD assessment test; CIS-FS: Checklist Individual Strength-fatigue subscale; CG: control group; EG: experimental group; FACIT-FS: Functional Assessment of Chronic Illness therapy–fatigue subscale; HADS: Hospital Anxiety and Depression scale (A – anxiety, D – depression); PPT-28: 28-item physical performance test; QMVC: quadriceps maximal voluntary contraction; SGRQ: St. George's Respiratory Questionnaire (S – symptoms, A- activities, I – impact, T- total); 1-minSTS: 1-minute sit-to-stand test; 6MWD: 6-minute walk distance.
Healthcare utilization and occurrence of exacerbations during the six-month follow-up were similar between groups. Detailed results are presented in the appendix (Table A13).
Adherence and occurrence of adverse eventsParticipants attended to 24[17–32] PA sessions (67[62–87]% attendance rate). Sixteen (80 %) participants delivered the diary completed after the six-month intervention (25[14–57.5] registries). Eight (40 %) participants reported walking outdoors once to three times per week (21[19–56.5] registries). Adverse events retrieved from diaries and possibly related to the community-based PAs were reported by three participants (15 %) during one PA session, two reported muscle soreness and one leg cramps. One participant slipped on the stairs while exiting the pool (without injury). A few months later the same participant complained about sciatic pain (unrelated to the previous fall), leading to the PA interruption. Another participant associated the occurrence of an exacerbation with the participation in the pool exercise class, which led to a change to another PA modality (senior exercise class).
DiscussionThis study showed that, six-months after completing PR, the PICk UP intervention was effective and efficacious in preventing PA decline in people with COPD. Sit-to-stand performance was also preserved in the PICk UP group. PR benefits on the remaining health-related outcome measures were sustained in both groups.
The PICk UP intervention was successful in maintaining the PA levels after PR independently of the outcome measure (objective/subjective) or analysis (ITT/per-protocol, adjusted/unadjusted) used. Our study differs from previous post-PR maintenance studies6 by allowing participants to choose and enrol in diverse PA modalities, aligning with the principle that individual preferences/needs vary and “one size does not fit all”.10,11,23,26 A good attendance rate was registered (67 %), surpassing attendance previously reported in PA maintenance studies14,18,36,39 and the typical 50 % rate seen in long-term treatments for chronic diseases.37 The social connections, supervision and peer-support,19,22,23,26 health professional support, home proximity and infrastructure convenience are well-known PA facilitators23,25,26 and might have contributed to our PA results.
Over twenty maintenance studies using PA-based interventions have been published,6-10 yet only a few reported PA as an outcome.14,20,39,40 Integrating PA as an endpoint in maintenance trials is crucial given its prognostic value in COPD.34 The only study measuring PA objectively14 observed similar findings to our study, i.e., in the EG the PA levels were preserved for the whole six-month period and in the CG a significant PA decline was present at six- but not at three-months.14 In fact, the decline noticed in steps/day in our CG exceeded the minimal clinically important difference established for the likelihood of medical events (350–1110 steps/day) in COPD.41 Therefore, our findings highlight the need for policy makers to support effective PA maintenance strategies.
The PICk UP and the PR programmes used in this study targeted PA but did not focus on reducing time spent in sedentary behaviour, which might explain the absence of improvements in sedentary lifestyle after both interventions.42,43 How to successfully reduce sedentary behaviour in COPD is still an enigma,44 however, it is worth noting, that our population spent an average of 400 min in sedentary behaviour, below the critical 510-min threshold tied to COPD mortality.45
Overall, six-months after PR completion, its benefits were still present in both groups, except in sit-to-stand performance, which declined in the CG. Our findings suggest that PA is likely to be the first benefit to decline after PR. A reduction in PA while exercise capacity (6MWT) remained stable has been previously observed in COPD.46 Several reviews have reported inconsistent findings regarding the effect of maintenance strategies on the 6MWT,6,7,9,10 with some reporting positive significant results at six-months,7,9 while others highlight the existent uncertainty in this field.6,10 Unlike the 6MWT, the 1-minSTS was not included as an outcome measure in previous maintenance reviews.6,7,9,10 Although an association between these instruments has been shown,47-49 the fact that daily mobility primarily involves walking-based activities whereas sit-to-stand or squat activities tend to occur less frequently throughout the day and are typically not repeated (unless for exercise purposes), may contribute to a faster decline measured by the 1-minSTS.
Health-related quality of life benefits were sustained independently of the PICk UP intervention. These findings are aligned with previous maintenance strategies reviews.6,7,9,10 In accordance with previous conclusions,6 the PICk UP did not provide any extra advantage in terms of health care utilization or prevention of exacerbations. Both groups experienced few exacerbations and used the healthcare system occasionally. Two factors could explain this: the six-month follow-up period may have been insufficient to assess these outcomes; most of our sample consisted of non-exacerbators (94 %), and it is known that exacerbation history is the most important predictor for future occurrences.50 Moreover, the decrease in PA in the CG, even with few exacerbations occurring during the follow-up period, reinforces the inconsistency noted in the connection between PA decline and exacerbation frequency.51
This study has several strengths. Firstly, it introduced a novel maintenance strategy that was personalised according to participants’ preferences and needs, embedded in their local context, and settled in an intersectoral partnership. To the best of the authors knowledge, this was the first study where participants could select and engage in various PA options, according to their preferences. Secondly, aligning with other PA interventions,21 the PICk UP intervention was safe, with only minor adverse events being reported. An additional strength of this study is its multicentre design with a rigorous methodology, including blinded assessors, and closely reflecting real-world conditions. Furthermore, the agreement between the ITT, per-protocol, adjusted and unadjusted analysis enhances confidence in our results.
Nevertheless, some limitations should be acknowledged. Firstly, effects of the PICk UP were only assessed over a medium-term period of six-months. A longer follow-up, ideally at least one year, is necessary to determine if the PICk UP programme was able to preserve PA levels in the EG, and whether other PR benefits were still maintained in both groups, in the long-term. Secondly, the possibility for participants of integrating different PA modalities added heterogeneity. Nevertheless, all PA modalities were properly validated and were mostly categorized as of moderate intensity32 (appendix). In addition, this study used the community real-world resources, therefore, the availability of PA options varied across city councils, resulting in some participants having a broader range of options compared to others. A sensitivity analysis based on type of PA chosen, city council or participants’ severity would have been relevant, however, the sample size was not large enough to perform it. The sample size may also have been insufficient to detect changes in the secondary outcomes (no power calculations were performed), therefore, caution is advised when interpreting these results. It is worth noting that the organisation, enrolment capacity, predisposition to accept and adherence to our study was affected by the COVID-19 pandemic. Although changes in organisation (e.g., physical distance among people, different entrances and exits) were similar across all site locations and adherence to the PICk UP intervention was considered good, the fear of COVID-19 may have precluded some participants from integrating community-based PAs. Another shortcoming of this study is related to the cutoff used to estimate MVPA,52 developed with healthy individuals and never validated in people with COPD. Our findings may not be generalizable to people with more severe COPD (GOLD grades 4 and E, and oxygen-dependent), or females, since these groups were underrepresented in our sample. Similarly, results might not apply to those recruited from inpatient or hospital-based settings, or failing to adhere to PR as only participants who completed an outpatient community-based PR programme were recruited. Finally, due to the nature of the intervention, blinding of participants was not possible. Currently, the Portuguese Health System does not cover the expenses related to community-based PAs. In this project, participants had free access to the entire PICk UP intervention, so the potential impact of financial constraints was not considered. Future studies performing a cost-analysis of the PICk UP intervention and assessing the efficacy/ effectiveness of this programme based on the type of PA are required to support its broader implementation and influence policy makers.
ConclusionsThe personalised community-based PA programme implemented was effective and efficacious in preventing a PA decline over a six-month period, following PR in people with COPD. Although there seems to have been a decline in sit-to-stand performance, this was counteracted with the PICk UP intervention. The remaining PR benefits seem to have lasted after six-months of PR, regardless of the maintenance strategy employed. Our findings highlight the importance of policymakers supporting sustainable PA maintenance strategies which bring the healthcare systems closer to patients’ communities and foster multisectoral partnerships to prevent the PA reduction after PR in people with COPD.
FundingThis work was funded by the project “CENTR(AR): pulmões em andamento” by Programa de Parcerias para o Impacto, Portugal Inovação Social through Programa Operacional Inclusão Social e Emprego (POISE-03-4639-FSE-000597); by Fundação para a Ciência e a Tecnologia (SFRH/BD/148738/2019 and COVID/BD/153476/2023) by Fundo Social Europeu through Programa Operacional Regional Centro, and by Programa Operacional Competitividade e Internacionalização (COMPETE 2020 - POCI-01-0145-FEDER-007628; UIDB/04501/2020 and UIDP/04501/2020).
The authors have no conflicts of interest to declare.
The authors would like to thank Guilherme Rodrigues, Cíntia Dias, Ana Grave, Samuel Santos, Maria Gomes, Inês Agostinho and Carolina Monteiro, all affiliated to the Respiratory Research and Rehabilitation Laboratory (Lab3R), School of Health Sciences, University of Aveiro, for their contribution during the data collection. In addition, we would like to thank the city councils and primary health care centres of Aveiro, Estarreja, Oliveira-do-Bairro and Montemor-o-Velho, for all the support provided to this project.